Microtissue engineered constructs with living axons for targeted nervous system reconstruction
- PMID: 22702955
- PMCID: PMC3482875
- DOI: 10.1089/ten.TEA.2011.0534
Microtissue engineered constructs with living axons for targeted nervous system reconstruction
Abstract
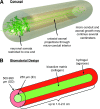
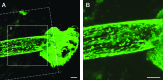
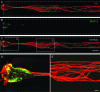
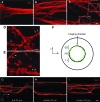
As a common feature of many neurological diseases and injury, the loss of axon pathways can have devastating effects on function. Here, we demonstrate a new strategy to restore damaged axon pathways using transplantable miniature constructs consisting of living neurons and axonal tracts internalized within hydrogel tubes. These hydrogel microconduits were developed through an iterative process to support neuronal survival and directed axon growth. The design included hollow agarose tubes providing a relatively stiff outer casing to direct constrained unidirectional outgrowth of axons through a central soft collagen matrix, with overall dimensions of 250 μm inner diameter ×500 μm outer diameter and extending up to several centimeters. The outer casing was also designed to provide structural support of neuronal/axonal cultures during transplantation of the construct. Using neuron culture conditions optimized for the microconduits, dissociated dorsal root ganglia neurons were seeded in the collagen at one end of the conduits. Over the following week, high-resolution confocal microscopy demonstrated that the neurons survived and the somata remained in a tight cluster at the original seeding site. In addition, robust outgrowth of axons from the neurons was found, with axon fascicles constrained in a longitudinal projection along the internal collagen canal and extending over 5 mm in length. Notably, this general geometry recapitulates the anatomy of axon tracts. As such, these constructs may be useful to repair damaged axon projections by providing a transplantable bridge of living axons. Moreover, the small size of the construct permits follow-on studies of minimally invasive transplantation into potentially sensitive regions of the nervous system.
Figures


Similar articles
-
Transplantable living scaffolds comprised of micro-tissue engineered aligned astrocyte networks to facilitate central nervous system regeneration.Acta Biomater. 2016 Jul 1;38:44-58. doi: 10.1016/j.actbio.2016.04.021. Epub 2016 Apr 29. Acta Biomater. 2016. PMID: 27090594 Free PMC article.
-
Tissue engineered axon-based "living scaffolds" promote survival of spinal cord motor neurons following peripheral nerve repair.J Tissue Eng Regen Med. 2020 Dec;14(12):1892-1907. doi: 10.1002/term.3145. Epub 2020 Dec 9. J Tissue Eng Regen Med. 2020. PMID: 33049797
-
Rebuilding Brain Circuitry with Living Micro-Tissue Engineered Neural Networks.Tissue Eng Part A. 2015 Nov;21(21-22):2744-56. doi: 10.1089/ten.TEA.2014.0557. Epub 2015 Oct 23. Tissue Eng Part A. 2015. PMID: 26414439 Free PMC article.
-
A novel neuroprosthetic interface with the peripheral nervous system using artificially engineered axonal tracts.Neurol Res. 2008 Dec;30(10):1063-7. doi: 10.1179/174313208X362541. Neurol Res. 2008. PMID: 19079981 Review.
-
The role of neurotrophic factors in nerve regeneration.Neurosurg Focus. 2009 Feb;26(2):E3. doi: 10.3171/FOC.2009.26.2.E3. Neurosurg Focus. 2009. PMID: 19228105 Review.
Cited by
-
Functional Cortical Axon Tracts Generated from Human Stem Cell-Derived Neurons.Tissue Eng Part A. 2019 May;25(9-10):736-745. doi: 10.1089/ten.TEA.2018.0270. Epub 2019 Mar 29. Tissue Eng Part A. 2019. PMID: 30648482 Free PMC article.
-
Living scaffolds for neuroregeneration.Curr Opin Solid State Mater Sci. 2014 Dec;18(6):308-318. doi: 10.1016/j.cossms.2014.07.004. Epub 2014 Sep 19. Curr Opin Solid State Mater Sci. 2014. PMID: 28736499 Free PMC article.
-
Intra-articular collagenase in the spinal facet joint induces pain, DRG neuron dysregulation and increased MMP-1 absent evidence of joint destruction.Sci Rep. 2020 Dec 15;10(1):21965. doi: 10.1038/s41598-020-78811-3. Sci Rep. 2020. PMID: 33319791 Free PMC article.
-
Innervation: the missing link for biofabricated tissues and organs.NPJ Regen Med. 2020 Jun 5;5:11. doi: 10.1038/s41536-020-0096-1. eCollection 2020. NPJ Regen Med. 2020. PMID: 32550009 Free PMC article. Review.
-
Engineering peripheral nerve repair.Curr Opin Biotechnol. 2013 Oct;24(5):887-92. doi: 10.1016/j.copbio.2013.05.006. Epub 2013 Jun 19. Curr Opin Biotechnol. 2013. PMID: 23790730 Free PMC article. Review.
References
-
- Tallantyre E.C. Bo L. Al-Rawashdeh O. Owens T. Polman C.H. Lowe J.S. Evangelou N. Clinico-pathological evidence that axonal loss underlies disability in progressive multiple sclerosis. Mult Scler. 2010;16:406. - PubMed
-
- Belal A. Ylikoski J. Pathology as it relates to ear surgery II. Labyrinthectomy. J Laryngol Otol. 1983;97:1. - PubMed
-
- Levin P.S. Newman S.A. Quigley H.A. Miller N.R. A clinicopathologic study of optic neuropathies associated with intracranial mass lesions with quantification of remaining axons. Am J Ophthalmol. 1983;95:295. - PubMed
-
- Marshall V.G. Bradley W.G., Jr. Marshall C.E. Bhoopat T. Rhodes R.H. Deep white matter infarction: correlation of MR imaging and histopathologic findings. Radiology. 1988;167:517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

