Microtubule S-glutathionylation as a potential approach for antimitotic agents
- PMID: 22703118
- PMCID: PMC3534152
- DOI: 10.1186/1471-2407-12-245
Microtubule S-glutathionylation as a potential approach for antimitotic agents
Abstract
Background: Microtubules have been one of the most effective targets for the development of anticancer agents. Cancer cells treated by these agents are characterized by cell arrest at G2/M phase. Microtubule-targeting drugs are, therefore, referred to as antimitotic agents. However, the clinical application of the current antimitotic drugs is hampered by emerging drug resistance which is the major cause of cancer treatment failure. The clinical success of antimitotic drugs and emerging drug resistance has prompted a search for new antimitotic agents, especially those with novel mechanisms of action. The aim of this study was to determine whether microtubules can be S-glutathionylated in cancer cells and whether the glutathionylation will lead to microtubule dysfunction and cell growth inhibition. The study will determine whether microtubule S-glutathionylation can be a novel approach for antimitotic agents.
Methods: 2-Acetylamino-3-[4-(2-acetylamino-2-carboxyethylsulfanylcarbonylamino)phenyl carbamoylsulfanyl]propionic acid (2-AAPA) was used as a tool to induce microtubule S-glutathionylation. UACC-62 cells, a human melanoma cell line, were used as a cancer cell model. A pull-down assay with glutathione S-transferase (GST)-agarose beads followed by Western blot analysis was employed to confirm microtubule S-glutathionylation. Immunofluorescence microscopy using a mouse monoclonal anti-α-tubulin-FITC was used to study the effect of the S-glutathionylation on microtubule function; mainly polymerization and depolymerization. Flow cytometry was employed to examine the effect of the S-glutathionylation on cell cycle distribution and apoptosis. Cell morphological change was followed through the use of a Zeiss AXIO Observer A1 microscope. Cancer cell growth inhibition by 2-AAPA was investigated with ten human cancer cell lines.
Results: Our investigation demonstrated that cell morphology was changed and microtubules were S-glutathionylated in the presence of 2-AAPA in UACC-62 cells. Accordingly, microtubules were found depolymerized and cells were arrested at G2/M phase. The affected cells were found to undergo apoptosis. Cancer growth inhibition experiments demonstrated that the concentrations of 2-AAPA required to produce the effects on microtubules were compatible to the concentrations producing cancer cell growth inhibition.
Conclusions: The data from this investigation confirms that microtubule S-glutathionylation leads to microtubule dysfunction and cell growth inhibition and can be a novel approach for developing antimitotic agents.
Figures
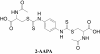





Similar articles
-
2-Acetylamino-3-[4-(2-acetylamino-2-carboxyethylsulfanylcarbonylamino) phenyl carbamoylsulfanyl] propionic acid, a glutathione reductase inhibitor, induces G2/M cell cycle arrest through generation of thiol oxidative stress in human esophageal cancer cells.Oncotarget. 2017 Jun 27;8(37):61846-61860. doi: 10.18632/oncotarget.18705. eCollection 2017 Sep 22. Oncotarget. 2017. PMID: 28977909 Free PMC article.
-
CWF-145, a novel synthetic quinolone derivative exerts potent antimitotic activity against human prostate cancer: Rapamycin enhances antimitotic drug-induced apoptosis through the inhibition of Akt/mTOR pathway.Chem Biol Interact. 2016 Dec 25;260:1-12. doi: 10.1016/j.cbi.2016.10.014. Epub 2016 Oct 18. Chem Biol Interact. 2016. PMID: 27769712
-
Viriditoxin Stabilizes Microtubule Polymers in SK-OV-3 Cells and Exhibits Antimitotic and Antimetastatic Potential.Mar Drugs. 2020 Aug 27;18(9):445. doi: 10.3390/md18090445. Mar Drugs. 2020. PMID: 32867174 Free PMC article.
-
How do microtubule-targeted drugs work? An overview.Curr Cancer Drug Targets. 2007 Dec;7(8):730-42. doi: 10.2174/156800907783220417. Curr Cancer Drug Targets. 2007. PMID: 18220533 Review.
-
[Antimitotic agents].Gan To Kagaku Ryoho. 1997 Sep;24(11):1519-25. Gan To Kagaku Ryoho. 1997. PMID: 9309150 Review. Japanese.
Cited by
-
Glyoxalase 2: Towards a Broader View of the Second Player of the Glyoxalase System.Antioxidants (Basel). 2022 Oct 28;11(11):2131. doi: 10.3390/antiox11112131. Antioxidants (Basel). 2022. PMID: 36358501 Free PMC article. Review.
-
Redox-Related Proteins in Melanoma Progression.Antioxidants (Basel). 2022 Feb 22;11(3):438. doi: 10.3390/antiox11030438. Antioxidants (Basel). 2022. PMID: 35326089 Free PMC article.
-
Metabolic Shades of S-D-Lactoylglutathione.Antioxidants (Basel). 2022 May 20;11(5):1005. doi: 10.3390/antiox11051005. Antioxidants (Basel). 2022. PMID: 35624868 Free PMC article. Review.
-
Targeting redox regulation to treat substance use disorder using N‐acetylcysteine.Eur J Neurosci. 2019 Aug;50(3):2538-2551. doi: 10.1111/ejn.14130. Epub 2018 Sep 24. Eur J Neurosci. 2019. PMID: 30144182 Free PMC article. Review.
-
Cysteine Glutathionylation Acts as a Redox Switch in Endothelial Cells.Antioxidants (Basel). 2019 Aug 16;8(8):315. doi: 10.3390/antiox8080315. Antioxidants (Basel). 2019. PMID: 31426416 Free PMC article. Review.
References
-
- Dustin P. Microtubules, 2nd totally rev. edn. Berlin; New York: Springer; 1984.
-
- Raff EC, editor. The role of multiple tubulin isoforms in cellular microtubule function. New York: John Wiley & Sons; 1994.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

