Mechanisms of mammalian iron homeostasis
- PMID: 22703180
- PMCID: PMC3572738
- DOI: 10.1021/bi300752r
Mechanisms of mammalian iron homeostasis
Abstract
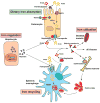
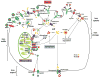
Iron is vital for almost all organisms because of its ability to donate and accept electrons with relative ease. It serves as a cofactor for many proteins and enzymes necessary for oxygen and energy metabolism, as well as for several other essential processes. Mammalian cells utilize multiple mechanisms to acquire iron. Disruption of iron homeostasis is associated with various human diseases: iron deficiency resulting from defects in the acquisition or distribution of the metal causes anemia, whereas iron surfeit resulting from excessive iron absorption or defective utilization causes abnormal tissue iron deposition, leading to oxidative damage. Mammals utilize distinct mechanisms to regulate iron homeostasis at the systemic and cellular levels. These involve the hormone hepcidin and iron regulatory proteins, which collectively ensure iron balance. This review outlines recent advances in iron regulatory pathways as well as in mechanisms underlying intracellular iron trafficking, an important but less studied area of mammalian iron homeostasis.
Figures


References
-
- Theil EC, Raymond KN. Bioinorganic chemistry. In: Bertini I, Gray HB, Lippard SJ, Valentine JS, editors. University Science Books; Mill Valley, CA: 1994. pp. 1–36.
-
- Wigglesworth JM, Baum H. In: In Iron in Biochemistry and Medicine II. Jacobs A, Worwood M, editors. Academic press; New York, NY: 1980. pp. 29–86.
-
- Pereira IAC, Teixeira M, Xavier AV. In: Bioinorganic chemistry: Trace element evolution from anaerobes to aerobes. Williams RJP, editor. Springer-Verlag; New York, NY: 2010. pp. 91–190.
-
- Nielands JB. Siderophores: structure and functions of microbial iron transport compounds. J Biol Chem. 1995;27:26723–26726. - PubMed
-
- Hider RC, Kong XL. Chemistry and biology of siderophores. Nat Products Rep. 2010;27:637–657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

