Mechanisms used by virulent Salmonella to impair dendritic cell function and evade adaptive immunity
- PMID: 22703384
- PMCID: PMC3449244
- DOI: 10.1111/j.1365-2567.2012.03614.x
Mechanisms used by virulent Salmonella to impair dendritic cell function and evade adaptive immunity
Abstract
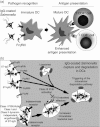
Innate and adaptive immunity are inter-related by dendritic cells (DCs), which directly recognize bacteria through the binding of pathogen-associated molecular patterns (PAMPs) to specialized receptors on their surface. After capturing and degrading bacteria, DCs present their antigens as small peptides bound to MHC molecules and prime naive bacteria-specific T cells. In response to PAMP recognition DCs undergo maturation, which is a phenotypic change that increases their immunogenicity and promotes the activation of naive T cells. As a result, a specific immune response that targets bacteria-derived antigens is initiated. Therefore, the characterization of DC-bacteria interactions is important to understand the mechanisms used by virulent bacteria to avoid adaptive immunity. Furthermore, any impairment of DC function might contribute to bacterial survival and dissemination inside the host. An example of a bacterial pathogen capable of interfering with DC function is Salmonella enterica serovar Typhimurium (S. Typhimurium). Virulent strains of this bacterium are able to differentially modulate the entrance to DCs, avoid lysosomal degradation and prevent antigen presentation on MHC molecules. These features of virulent S. Typhimurium are controlled by virulence proteins, which are encoded by pathogenicity islands. Modulation of DC functions by these gene products is supported by several studies showing that pathogenesis might depend on this attribute of virulent S. Typhimurium. Here we discuss some of the recent data reported by the literature showing that several virulence proteins from Salmonella are required to modulate DC function and the activation of host adaptive immunity.
© 2012 The Authors. Immunology © 2012 Blackwell Publishing Ltd.
Figures

References
-
- Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–6. - PubMed
-
- Rescigno M. Dendritic cells and the complexity of microbial infection. Trends Microbiol. 2002;10:425–61. - PubMed
-
- Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

