Synthesis and biological activity of substituted 2,4-diaminopyrimidines that inhibit Bacillus anthracis
- PMID: 22703705
- PMCID: PMC3408765
- DOI: 10.1016/j.ejmech.2012.05.018
Synthesis and biological activity of substituted 2,4-diaminopyrimidines that inhibit Bacillus anthracis
Abstract
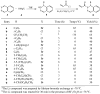
A series of substituted 2,4-diaminopyrimidines 1 has been prepared and evaluated for activity against Bacillus anthracis using previously reported (±)-3-{5-[(2,4-diamino-5-pyrimidinyl)methyl]-2,3-dimethoxyphenyl}-1-(1-propyl-2(1H)-phthalazinyl)-2-propen-1-one (1a), with a minimum inhibitory concentration (MIC) value of 1-3 μg/mL, as the standard. In the current work, the corresponding isobutenyl (1e) and phenyl (1h) derivatives displayed the most significant activity in terms of the lowest MICs with values of 0.5 μg/mL and 0.375-1.5 μg/mL, respectively. It is likely that the S isomers of 1 will bind the substrate-binding pocket of dihydrofolate reductase (DHFR) as in B. anthracis was found for (S)-1a. The final step in the convergent synthesis of target systems 1 from (±)-1-(1-substituted-2(1H)-phthalazinyl)-2-propen-1-ones 6 with 2,4-diamino-5-(5-iodo-3,4-dimethoxybenzyl)pyrimidine (13) was accomplished via a novel Heck coupling reaction under sealed-tube conditions.
Copyright © 2012 Elsevier Masson SAS. All rights reserved.
Figures


References
-
- Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Friedlander AM, Hauer J, McDade J, Osterholm MT, O’Toole T, Parker G, Perl TM, Russell PK, Tonat K. Anthrax as a biological weapon: Medical and public health management. Working group on civilian biodefense. JAMA. 1999;281:1735–1745. - PubMed
-
- Bourne CR, Bunce RA, Bourne PC, Berlin KD, Barrow EW, Barrow WW. Crystal structure of Bacillus anthracis dihydrofolate reductase with the dihydrophthalazine-based trimethoprim derivative RAB1 provides a structural explanation of potency and selectivity. Antimicrob Agents Chemother. 2009;53:3065–3073. - PMC - PubMed
-
- Jackson RC. In: In Antifolate Drugs in Cancer Therapy. Jackman AL, editor. Humana Press; Totowa, NJ: 1999.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

