Bone marrow transplantation increases efficacy of central nervous system-directed enzyme replacement therapy in the murine model of globoid cell leukodystrophy
- PMID: 22704480
- PMCID: PMC3444533
- DOI: 10.1016/j.ymgme.2012.05.021
Bone marrow transplantation increases efficacy of central nervous system-directed enzyme replacement therapy in the murine model of globoid cell leukodystrophy
Abstract
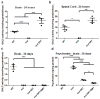
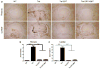
Globoid cell leukodystrophy (GLD, Krabbe disease), is an autosomal recessive, neurodegenerative disease caused by the deficiency of the lysosomal enzyme galactocerebrosidase (GALC). In the absence of GALC, the toxic metabolite psychosine accumulates in the brain and causes the death of the myelin-producing cells, oligodendrocytes. Currently, the only therapy for GLD is hematopoietic stem cell transplantation using bone marrow (BMT) or umbilical cord blood. However, this is only partially effective. Previous studies have shown that enzyme replacement therapy (ERT) provides some therapeutic benefit in the murine model of GLD, the Twitcher mouse. Experiments have also shown that two disparate therapies can produce synergistic effects when combined. The current study tests the hypothesis that BMT will increase the therapeutic effects of ERT when these two treatments are combined. Twitcher mice were treated with either ERT alone or both ERT and BMT during the first 2-4 days of life. Recombinant enzyme was delivered by intracerebroventricular (ICV) and intrathecal (IT) injections. Twitcher mice receiving ERT had supraphysiological levels of GALC activity in the brain 24h after injection. At 36 days of age, ERT-treated Twitcher mice had reduced psychosine levels, reduced neuroinflammation, improved motor function, and increased lifespan. Twitcher mice receiving both ERT and BMT had significantly increased lifespan, improved motor function, reduced psychosine levels, and reduced neuroinflammation in certain areas of the brain compared to untreated or ERT-treated Twitcher mice. Together, these results indicate that BMT enhances the efficacy of ERT in GLD.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Mechanism-based combination treatment dramatically increases therapeutic efficacy in murine globoid cell leukodystrophy.J Neurosci. 2015 Apr 22;35(16):6495-505. doi: 10.1523/JNEUROSCI.4199-14.2015. J Neurosci. 2015. PMID: 25904800 Free PMC article.
-
CNS-targeted AAV5 gene transfer results in global dispersal of vector and prevention of morphological and function deterioration in CNS of globoid cell leukodystrophy mouse model.Mol Genet Metab. 2011 Aug;103(4):367-77. doi: 10.1016/j.ymgme.2011.05.005. Epub 2011 May 12. Mol Genet Metab. 2011. PMID: 21620749
-
Bone marrow transplantation augments the effect of brain- and spinal cord-directed adeno-associated virus 2/5 gene therapy by altering inflammation in the murine model of globoid-cell leukodystrophy.J Neurosci. 2011 Jul 6;31(27):9945-57. doi: 10.1523/JNEUROSCI.1802-11.2011. J Neurosci. 2011. PMID: 21734286 Free PMC article.
-
Insights into the Pathogenesis and Treatment of Krabbe Disease.Pediatr Endocrinol Rev. 2016 Jun;13 Suppl 1:689-96. Pediatr Endocrinol Rev. 2016. PMID: 27491217 Review.
-
Treatment for Krabbe's disease: Finding the combination.J Neurosci Res. 2016 Nov;94(11):1126-37. doi: 10.1002/jnr.23822. J Neurosci Res. 2016. PMID: 27638598 Free PMC article. Review.
Cited by
-
Cellular transplant therapies for globoid cell leukodystrophy: Preclinical and clinical observations.J Neurosci Res. 2016 Nov;94(11):1180-8. doi: 10.1002/jnr.23782. J Neurosci Res. 2016. PMID: 27638602 Free PMC article. Review.
-
New therapeutic approaches for Krabbe disease: The potential of pharmacological chaperones.J Neurosci Res. 2016 Nov;94(11):1203-19. doi: 10.1002/jnr.23762. J Neurosci Res. 2016. PMID: 27638604 Free PMC article. Review.
-
Preclinical studies in Krabbe disease: A model for the investigation of novel combination therapies for lysosomal storage diseases.Mol Ther. 2023 Jan 4;31(1):7-23. doi: 10.1016/j.ymthe.2022.09.017. Epub 2022 Oct 4. Mol Ther. 2023. PMID: 36196048 Free PMC article. Review.
-
Conditions for combining gene therapy with bone marrow transplantation in murine Krabbe disease.Bioimpacts. 2020;10(2):105-115. doi: 10.34172/bi.2020.13. Epub 2020 Mar 24. Bioimpacts. 2020. PMID: 32363154 Free PMC article.
-
Combined gene/cell therapies provide long-term and pervasive rescue of multiple pathological symptoms in a murine model of globoid cell leukodystrophy.Hum Mol Genet. 2015 Jun 15;24(12):3372-89. doi: 10.1093/hmg/ddv086. Epub 2015 Mar 5. Hum Mol Genet. 2015. PMID: 25749991 Free PMC article.
References
-
- Wenger DA, Rafi MA, Luzi P. Molecular genetics of Krabbe disease (globoid cell leukodystrophy): diagnostic and clinical implications. Hum Mutat. 1997;10:268–279. - PubMed
-
- Wenger DA, Suzuki K, Suzuki Y, Suzuki K. Galactosylceramide lipidosis. Globoid cell leukodystrophy (Krabbe disease) In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B, editors. The Metabolic and Molecular Bases of Inherited Disease. 8. McGraw-Hill; New York: 2001. pp. 3669–3687.
-
- Haq E, Giri S, Singh I, Singh AK. Molecular mechanism of psychosine-induced cell death in human oligodendrocyte cell line. J Neurochem. 2003;86:1428–1440. - PubMed
-
- Taniike M, Mohri I, Eguchi N, Irikura D, Urade Y, Okada S, Suzuki K. An apoptotic depletion of oligodendrocytes in the twitcher, a murine model of globoid cell leukodystrophy. J Neuropathol Exp Neurol. 1999;58:644–653. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

