Plasmacytoid dendritic cells are productively infected and activated through TLR-7 early after arenavirus infection
- PMID: 22704622
- PMCID: PMC3377983
- DOI: 10.1016/j.chom.2012.04.017
Plasmacytoid dendritic cells are productively infected and activated through TLR-7 early after arenavirus infection
Abstract
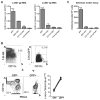
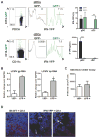
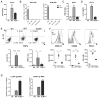
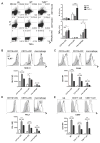
The antiviral response is largely mediated by dendritic cells (DCs), including conventional (c) DCs that function as antigen-presenting cells, and plasmacytoid (p) DCs that produce type I interferons, making them an attractive target for viruses. We find that the Old World arenaviruses lymphocytic choriomeningitis virus clone 13 (LCMV Cl13) and Lassa virus bind pDCs to a greater extent than cDCs. Consistently, LCMV Cl13 targets pDCs early after in vivo infection of its natural murine host and establishes a productive and robust replication cycle. pDCs coproduce type I interferons and proinflammatory cytokines, with the former being induced in both infected and uninfected pDCs, demonstrating a dissociation from intrinsic virus replication. TLR7 globally mediates pDC responses, limits pDC viral load, and promotes rapid innate and adaptive immune cell activation. These early events likely help dictate the outcome of infections with arenaviruses and other DC-replicating viruses and shed light on potential therapeutic targets.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Lymphocyte choriomeningitis virus plays hide-and-seek with type 1 interferon.Cell Host Microbe. 2012 Jun 14;11(6):553-5. doi: 10.1016/j.chom.2012.05.007. Cell Host Microbe. 2012. PMID: 22704613 Review.
References
-
- Baize S, Kaplon J, Faure C, Pannetier D, Georges-Courbot MC, Deubel V. Lassa virus infection of human dendritic cells and macrophages is productive but fails to activate cells. J Immunol. 2004;172:2861–2869. - PubMed
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Barton LL, Mets MB, Beauchamp CL. Lymphocytic choriomeningitis virus: emerging fetal teratogen. Am J Obstet Gynecol. 2002;187:1715–1716. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

