Timing and magnitude of type I interferon responses by distinct sensors impact CD8 T cell exhaustion and chronic viral infection
- PMID: 22704623
- PMCID: PMC3572910
- DOI: 10.1016/j.chom.2012.05.003
Timing and magnitude of type I interferon responses by distinct sensors impact CD8 T cell exhaustion and chronic viral infection
Abstract
Type I interferon (IFN-I) promotes antiviral CD8(+)T cell responses, but the contribution of different IFN-I sources and signaling pathways are ill defined. While plasmacytoid dendritic cells (pDCs) produce IFN-I upon TLR stimulation, IFN-I is induced in most cells by helicases like MDA5. Using acute and chronic lymphocytic choriomeningitis virus (LCMV) infection models, we determined that pDCs transiently produce IFN-I that minimally impacts CD8(+)T cell responses and viral persistence. Rather, MDA5 is the key sensor that induces IFN-I required for CD8(+)T cell responses. In the absence of MDA5, CD8(+)T cell responses to acute infection rely on CD4(+)T cell help, and loss of both CD4(+)T cells and MDA5 results in CD8(+)T cell exhaustion and persistent infection. Chronic LCMV infection rapidly attenuates IFN-I responses, but early administration of exogenous IFN-I rescues CD8(+)T cells, promoting viral clearance. Thus, effective antiviral CD8(+)T cell responses depend on the timing and magnitude of IFN-I production.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no further conflicts of interest.
Figures


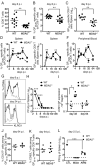


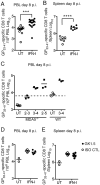
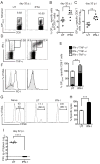
Comment in
-
Lymphocyte choriomeningitis virus plays hide-and-seek with type 1 interferon.Cell Host Microbe. 2012 Jun 14;11(6):553-5. doi: 10.1016/j.chom.2012.05.007. Cell Host Microbe. 2012. PMID: 22704613
References
-
- Audige A, Hofer U, Dittmer U, van den Broek M, Speck RF. Evaluation of the immunomodulatory and antiviral effects of the cytokine combination IFN-alpha and IL-7 in the lymphocytic choriomeningitis virus and Friend retrovirus mouse infection models. Viral Immunol. 2011;24:375–385. - PubMed
-
- Barbalat R, Ewald SE, Mouchess ML, Barton GM. Nucleic acid recognition by the innate immune system. Annu Rev Immunol. 2011;29:185–214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

