Monitoring the inflammatory response to infection through the integration of MALDI IMS and MRI
- PMID: 22704626
- PMCID: PMC3377982
- DOI: 10.1016/j.chom.2012.04.018
Monitoring the inflammatory response to infection through the integration of MALDI IMS and MRI
Abstract
Systemic bacterial infection is characterized by a robust whole-organism inflammatory response. Analysis of the immune response to infection involves technologies that typically focus on single organ systems and lack spatial information. Additionally, the analysis of individual inflammatory proteins requires antibodies specific to the protein of interest, limiting the panel of proteins that can be analyzed. Herein we describe the application of matrix-assisted laser desorption/ionization imaging mass spectrometry (MALDI IMS) to mice systemically infected with Staphylococcus aureus to identify inflammatory protein masses that respond to infection throughout an entire infected animal. Integrating the resolution afforded by magnetic resonance imaging (MRI) with the sensitivity of MALDI IMS provides three-dimensional spatially resolved information regarding the distribution of innate immune proteins during systemic infection, allowing comparisons to in vivo structural information and soft-tissue contrast via MRI. Thus, integrating MALDI IMS with MRI provides a systems-biology approach to study inflammation during infection.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflicts of interest to report.
Figures

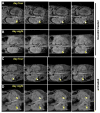


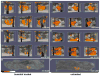
Comment in
-
Inflammation in 3D.Cell Host Microbe. 2012 Jun 14;11(6):557-9. doi: 10.1016/j.chom.2012.05.006. Cell Host Microbe. 2012. PMID: 22704615
References
-
- Andersson M, Groseclose MR, Deutch AY, Caprioli RM. Imaging mass spectrometry of proteins and peptides: 3D volume reconstruction. Nature Methods. 2008;5:101–108. - PubMed
-
- Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, Torres VJ, Anderson KL, Dattilo BM, Dunman PM, Gerads R, et al. Metal Chelation and Inhibition of Bacterial Growth in Tissue Abscesses. Science. 2008;319:962–965. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

