Stochastic, adaptive sampling of information by microvilli in fly photoreceptors
- PMID: 22704990
- PMCID: PMC3420010
- DOI: 10.1016/j.cub.2012.05.047
Stochastic, adaptive sampling of information by microvilli in fly photoreceptors
Abstract
Background: In fly photoreceptors, light is focused onto a photosensitive waveguide, the rhabdomere, consisting of tens of thousands of microvilli. Each microvillus is capable of generating elementary responses, quantum bumps, in response to single photons using a stochastically operating phototransduction cascade. Whereas much is known about the cascade reactions, less is known about how the concerted action of the microvilli population encodes light changes into neural information and how the ultrastructure and biochemical machinery of photoreceptors of flies and other insects evolved in relation to the information sampling and processing they perform.
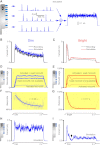
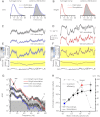
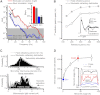
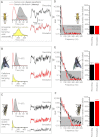
Results: We generated biophysically realistic fly photoreceptor models, which accurately simulate the encoding of visual information. By comparing stochastic simulations with single cell recordings from Drosophila photoreceptors, we show how adaptive sampling by 30,000 microvilli captures the temporal structure of natural contrast changes. Following each bump, individual microvilli are rendered briefly (~100-200 ms) refractory, thereby reducing quantum efficiency with increasing intensity. The refractory period opposes saturation, dynamically and stochastically adjusting availability of microvilli (bump production rate: sample rate), whereas intracellular calcium and voltage adapt bump amplitude and waveform (sample size). These adapting sampling principles result in robust encoding of natural light changes, which both approximates perceptual contrast constancy and enhances novel events under different light conditions, and predict information processing across a range of species with different visual ecologies.
Conclusions: These results clarify why fly photoreceptors are structured the way they are and function as they do, linking sensory information to sensory evolution and revealing benefits of stochasticity for neural information processing.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures


Comment in
-
Visual transduction: microvilli orchestrate photoreceptor responses to light.Curr Biol. 2012 Aug 7;22(15):R595-7. doi: 10.1016/j.cub.2012.07.001. Curr Biol. 2012. PMID: 22877777
Similar articles
-
A biomimetic fly photoreceptor model elucidates how stochastic adaptive quantal sampling provides a large dynamic range.J Physiol. 2017 Aug 15;595(16):5439-5456. doi: 10.1113/JP273614. Epub 2017 May 17. J Physiol. 2017. PMID: 28369994 Free PMC article. Review.
-
Refractory sampling links efficiency and costs of sensory encoding to stimulus statistics.J Neurosci. 2014 May 21;34(21):7216-37. doi: 10.1523/JNEUROSCI.4463-13.2014. J Neurosci. 2014. PMID: 24849356 Free PMC article.
-
How a fly photoreceptor samples light information in time.J Physiol. 2017 Aug 15;595(16):5427-5437. doi: 10.1113/JP273645. Epub 2017 Apr 17. J Physiol. 2017. PMID: 28233315 Free PMC article. Review.
-
Random Photon Absorption Model Elucidates How Early Gain Control in Fly Photoreceptors Arises from Quantal Sampling.Front Comput Neurosci. 2016 Jun 24;10:61. doi: 10.3389/fncom.2016.00061. eCollection 2016. Front Comput Neurosci. 2016. PMID: 27445779 Free PMC article.
-
Visual transduction: microvilli orchestrate photoreceptor responses to light.Curr Biol. 2012 Aug 7;22(15):R595-7. doi: 10.1016/j.cub.2012.07.001. Curr Biol. 2012. PMID: 22877777
Cited by
-
Postembryonic developmental changes in photoreceptors of the stick insect Carausius morosus enhance the shift to an adult nocturnal life-style.J Neurosci. 2012 Nov 21;32(47):16821-31. doi: 10.1523/JNEUROSCI.2612-12.2012. J Neurosci. 2012. PMID: 23175835 Free PMC article.
-
Evidence for Dynamic Network Regulation of Drosophila Photoreceptor Function from Mutants Lacking the Neurotransmitter Histamine.Front Neural Circuits. 2016 Mar 22;10:19. doi: 10.3389/fncir.2016.00019. eCollection 2016. Front Neural Circuits. 2016. PMID: 27047343 Free PMC article.
-
Channel identification machines.Comput Intell Neurosci. 2012;2012:209590. doi: 10.1155/2012/209590. Epub 2012 Nov 14. Comput Intell Neurosci. 2012. PMID: 23227035 Free PMC article.
-
Difference in dynamic properties of photoreceptors in a butterfly, Papilio xuthus: possible segregation of motion and color processing.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2015 Dec;201(12):1115-23. doi: 10.1007/s00359-015-1039-y. Epub 2015 Sep 2. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2015. PMID: 26329322
-
Performance of blue- and green-sensitive photoreceptors of the cricket Gryllus bimaculatus.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2014 Mar;200(3):209-19. doi: 10.1007/s00359-013-0879-6. Epub 2014 Jan 8. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2014. PMID: 24398538
References
-
- van Hateren J.H. Processing of natural time series of intensities by the visual system of the blowfly. Vision Res. 1997;37:3407–3416. - PubMed
-
- Hardie R.C., Postma M. Phototransduction in microvillar photoreceptors of Drosophila and other invertebrates. In: Basbaum A.I., Kaneko A., Shepherd G.M., Westheimer G., editors. Volume 1. Academic Press; San Diego: 2008. pp. 77–130. (The Senses: A Comprehensive Reference. Vision).
-
- van Hateren J.H., Snippe H.P. Information theoretical evaluation of parametric models of gain control in blowfly photoreceptor cells. Vision Res. 2001;41:1851–1865. - PubMed
-
- Nikolic K., Loizu J., Degenaar P., Toumazou C. A stochastic model of the single photon response in Drosophila photoreceptors. Integr Biol (Camb) 2010;2:354–370. - PubMed
Publication types
MeSH terms
Grants and funding
- BB/H013849/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- E19850/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/F012071/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/D001900/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/G006865/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

