The VHL/HIF axis in clear cell renal carcinoma
- PMID: 22705278
- PMCID: PMC3663044
- DOI: 10.1016/j.semcancer.2012.06.001
The VHL/HIF axis in clear cell renal carcinoma
Abstract
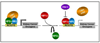
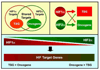
Inactivation of the VHL tumor suppressor protein (pVHL) is a common event in clear cell renal carcinoma, which is the most common form of kidney cancer. pVHL performs many functions, including serving as the substrate recognition module of an ubiquitin ligase complex that targets the alpha subunits of the heterodimeric HIF transcription factor for proteasomal degradation. Deregulation of HIF2α appears to be a driving force in pVHL-defective clear cell renal carcinomas. In contrast, genetic and functional studies suggest that HIF1α serves as a tumor suppressor and is a likely target of the 14q deletions that are characteristic of this tumor type. Drugs that inhibit HIF2α, or its downstream targets such as VEGF, are in various stages of clinical testing. Indeed, clear cell renal carcinomas are exquisitely sensitive to VEGF deprivation and four VEGF inhibitors have now been approved for the treatment of this disease.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures


References
-
- Maher E, Kaelin WG. von Hippel-Lindau Disease. Medicine. 1997;76:381–391. - PubMed
-
- Kim WY, Kaelin WG. Role of VHL Gene Mutation in Human Cancer. J Clin Onc. 2004;22:4991–5004. - PubMed
-
- Furge KA, Tan MH, Dykema K, Kort E, Stadler W, Yao X, et al. Identification of deregulated oncogenic pathways in renal cell carcinoma: an integrated oncogenomic approach based on gene expression profiling. Oncogene. 2007;26:1346–1350. - PubMed
-
- Mandriota SJ, Turner KJ, Davies DR, Murray PG, Morgan NV, Sowter HM, et al. HIF activation identifies early lesions in VHL kidneys: evidence for site-specific tumor suppressor function in the nephron. Cancer Cell. 2002;1:459–468. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

