The role of fatty acid amide hydrolase inhibition in nicotine reward and dependence
- PMID: 22705310
- PMCID: PMC3477273
- DOI: 10.1016/j.lfs.2012.05.015
The role of fatty acid amide hydrolase inhibition in nicotine reward and dependence
Abstract
The endogenous cannabinoid anandamide (AEA) exerts the majority of its effects at CB1 and CB2 receptors and is degraded by fatty acid amide hydrolase (FAAH). FAAH KO mice and animals treated with FAAH inhibitors are impaired in their ability to hydrolyze AEA and other non-cannabinoid lipid signaling molecules, such as oleoylethanolamide (OEA) and palmitoylethanolamide (PEA). AEA and these other substrates activate non-cannabinoid receptor systems, including TRPV1 and PPAR-α receptors. In this mini review, we describe the functional consequences of FAAH inhibition on nicotine reward and dependence as well as the underlying endocannabinoid and non-cannabinoid receptor systems mediating these effects. Interestingly, FAAH inhibition seems to mediate nicotine dependence differently in mice and rats. Indeed, pharmacological and genetic FAAH disruption in mice enhances nicotine reward and withdrawal. However, in rats, pharmacological blockade of FAAH significantly inhibits nicotine reward and has no effect in nicotine withdrawal. Studies suggest that non-cannabinoid mechanisms may play a role in these species differences.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
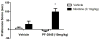
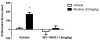
Similar articles
-
Endogenous fatty acid ethanolamides suppress nicotine-induced activation of mesolimbic dopamine neurons through nuclear receptors.J Neurosci. 2008 Dec 17;28(51):13985-94. doi: 10.1523/JNEUROSCI.3221-08.2008. J Neurosci. 2008. PMID: 19091987 Free PMC article.
-
The endogenous cannabinoid system modulates nicotine reward and dependence.J Pharmacol Exp Ther. 2008 Aug;326(2):483-92. doi: 10.1124/jpet.108.138321. Epub 2008 May 1. J Pharmacol Exp Ther. 2008. PMID: 18451315 Free PMC article.
-
The anandamide transport inhibitor AM404 reduces the rewarding effects of nicotine and nicotine-induced dopamine elevations in the nucleus accumbens shell in rats.Br J Pharmacol. 2012 Apr;165(8):2539-48. doi: 10.1111/j.1476-5381.2011.01467.x. Br J Pharmacol. 2012. PMID: 21557729 Free PMC article.
-
Fatty acid amide hydrolase inhibitors--progress and potential.CNS Neurol Disord Drug Targets. 2011 Aug;10(5):545-58. doi: 10.2174/187152711796234989. CNS Neurol Disord Drug Targets. 2011. PMID: 21631410 Review.
-
Fatty acid amide hydrolase: a gate-keeper of the endocannabinoid system.Subcell Biochem. 2008;49:101-32. doi: 10.1007/978-1-4020-8831-5_4. Subcell Biochem. 2008. PMID: 18751909 Review.
Cited by
-
Cannabinoid Receptor 1 and Fatty Acid Amide Hydrolase Contribute to Operant Sensation Seeking in Mice.Int J Mol Sci. 2017 Jul 27;18(8):1635. doi: 10.3390/ijms18081635. Int J Mol Sci. 2017. PMID: 28749428 Free PMC article.
-
The cannabinoid CB2 receptor is necessary for nicotine-conditioned place preference, but not other behavioral effects of nicotine in mice.Psychopharmacology (Berl). 2013 Oct;229(4):591-601. doi: 10.1007/s00213-013-3117-6. Epub 2013 May 8. Psychopharmacology (Berl). 2013. PMID: 23652588 Free PMC article.
-
Footshock-Induced Abstinence from Compulsive Methamphetamine Self-administration in Rat Model Is Accompanied by Increased Hippocampal Expression of Cannabinoid Receptors (CB1 and CB2).Mol Neurobiol. 2022 Feb;59(2):1238-1248. doi: 10.1007/s12035-021-02656-8. Epub 2022 Jan 3. Mol Neurobiol. 2022. PMID: 34978045 Free PMC article.
-
Effects of Fatty Acid Amide Hydrolase (FAAH) Inhibitors in Non-Human Primate Models of Nicotine Reward and Relapse.Neuropsychopharmacology. 2015 Aug;40(9):2185-97. doi: 10.1038/npp.2015.62. Epub 2015 Mar 10. Neuropsychopharmacology. 2015. PMID: 25754762 Free PMC article.
-
Druggable Targets in Endocannabinoid Signaling.Adv Exp Med Biol. 2020;1274:177-201. doi: 10.1007/978-3-030-50621-6_8. Adv Exp Med Biol. 2020. PMID: 32894511 Free PMC article. Review.
References
-
- Ahern GP. Activation of TRPV1 by the satiety factor oleoylethanolamide. J Biol Chem. 2003 Aug 15;278(33):30429–34. - PubMed
-
- Ahn K, Smith SE, Liimatta MB, Beidler D, Sadagopan N, Dudley DT, Young T, Wren P, Zhang Y, Swaney S, Van Becelaere K, Blankman JL, Nomura DK, Bhattachar SN, Stiff C, Nomanbhoy TK, Weerapana E, Johnson DS, Cravatt BF. Mechanistic and pharmacological characterization of PF-04457845: a highly potent and selective fatty acid amide hydrolase inhibitor that reduces inflammatory and noninflammatory pain. J Pharmacol Exp Ther. 2011;338:114–24. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

