Cyclic di-GMP sensing via the innate immune signaling protein STING
- PMID: 22705373
- PMCID: PMC3697849
- DOI: 10.1016/j.molcel.2012.05.029
Cyclic di-GMP sensing via the innate immune signaling protein STING
Abstract
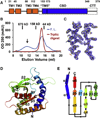
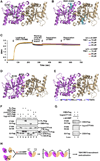
Detection of foreign materials is the first step of successful immune responses. Stimulator of interferon genes (STING) was shown to directly bind cyclic diguanylate monophosphate (c-di-GMP), a bacterial second messenger, and to elicit strong interferon responses. Here we elucidate the structural features of the cytosolic c-di-GMP binding domain (CBD) of STING and its complex with c-di-GMP. The CBD exhibits an α + β fold and is a dimer in the crystal and in solution. Surprisingly, one c-di-GMP molecule binds to the central crevice of a STING dimer, using a series of stacking and hydrogen bonding interactions. We show that STING is autoinhibited by an intramolecular interaction between the CBD and the C-terminal tail (CTT) and that c-di-GMP releases STING from this autoinhibition by displacing the CTT. The structures provide a remarkable example of pathogen-host interactions in which a unique microbial molecule directly engages the innate immune system.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



References
-
- Akira S, Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. - PubMed
-
- Barends TR, Hartmann E, Griese JJ, Beitlich T, Kirienko NV, Ryjenkov DA, Reinstein J, Shoeman RL, Gomelsky M, Schlichting I. Structure and mechanism of a bacterial light-regulated cyclic nucleotide phosphodiesterase. Nature. 2009;459:1015–1018. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

