Testis development requires the repression of Wnt4 by Fgf signaling
- PMID: 22705479
- PMCID: PMC3634333
- DOI: 10.1016/j.ydbio.2012.06.009
Testis development requires the repression of Wnt4 by Fgf signaling
Abstract
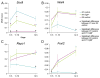
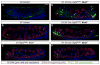
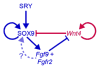
The bipotential gonad expresses genes associated with both the male and female pathways. Adoption of the male testicular fate is associated with the repression of many female genes including Wnt4. However, the importance of repression of Wnt4 to the establishment of male development was not previously determined. Deletion of either Fgf9 or Fgfr2 in an XY gonad resulted in up-regulation of Wnt4 and male-to-female sex reversal. We investigated whether the deletion if Wnt4 could rescue sex reversal in Fgf9 and Fgfr2 mutants. XY Fgf9/Wnt4 and Fgfr2/Wnt4 double mutants developed testes with male somatic and germ cells present, suggesting that the primary role of Fgf signaling is the repression of female-promoting genes. Thus, the decision to adopt the male fate is based not only on whether male genes, such as Sox9, are expressed, but also on the active repression of female genes, such as Wnt4. Because loss of Wnt4 results in the up-regulation of Fgf9, we also tested the possibility that derepression of Fgf9 was responsible for the aspects of male development observed in XX Wnt4 mutants. However, we found that the relationship between these two signaling factors is not symmetric: loss of Fgf9 in XX Wnt4(-/-) gonads does not rescue their partial female-to-male sex-reversal.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




References
-
- Bagheri-Fam S, Sim H, Bernard P, Jayakody I, Taketo MM, Scherer G, Harley VR. Loss of Fgfr2 leads to partial XY sex reversal. Dev Biol. 2008;314:71–83. - PubMed
-
- Barrios F, Filipponi D, Pellegrini M, Paronetto MP, Di Siena S, Geremia R, Rossi P, De Felici M, Jannini EA, Dolci S. Opposing effects of retinoic acid and Fgf9 on Nanos2 expression and meiotic entry of mouse germ cells. J Cell Sci. 2010;123:871–880. - PubMed
-
- Barske LA, Capel B. Estrogen represses SOX9 during sex determination in the red-eared slider turtle Trachemys scripta. Dev Biol. 2010;341:305–314. - PubMed
-
- Bernard P, Ryan J, Sim H, Czech DP, Sinclair AH, Koopman P, Harley VR. Wnt signaling in ovarian development inhibits Sf1 activation of Sox9 via the Tesco enhancer. Endocrinology 2011 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

