Nucleotide excision DNA repair is associated with age-related vascular dysfunction
- PMID: 22705887
- PMCID: PMC3430727
- DOI: 10.1161/CIRCULATIONAHA.112.104380
Nucleotide excision DNA repair is associated with age-related vascular dysfunction
Abstract
Background: Vascular dysfunction in atherosclerosis and diabetes mellitus, as observed in the aging population of developed societies, is associated with vascular DNA damage and cell senescence. We hypothesized that cumulative DNA damage during aging contributes to vascular dysfunction.
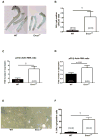
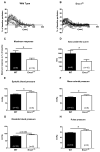
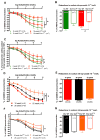
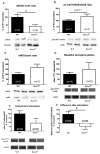
Methods and results: In mice with genomic instability resulting from the defective nucleotide excision repair genes ERCC1 and XPD (Ercc1(d/-) and Xpd(TTD) mice), we explored age-dependent vascular function compared with that in wild-type mice. Ercc1(d/-) mice showed increased vascular cell senescence, accelerated development of vasodilator dysfunction, increased vascular stiffness, and elevated blood pressure at a very young age. The vasodilator dysfunction was due to decreased endothelial nitric oxide synthase levels and impaired smooth muscle cell function, which involved phosphodiesterase activity. Similar to Ercc1(d/-) mice, age-related endothelium-dependent vasodilator dysfunction in Xpd(TTD) animals was increased. To investigate the implications for human vascular disease, we explored associations between single-nucleotide polymorphisms of selected nucleotide excision repair genes and arterial stiffness within the AortaGen Consortium and found a significant association of a single-nucleotide polymorphism (rs2029298) in the putative promoter region of DDB2 gene with carotid-femoral pulse wave velocity.
Conclusions: Mice with genomic instability recapitulate age-dependent vascular dysfunction as observed in animal models and in humans but with an accelerated progression compared with wild-type mice. In addition, we found associations between variations in human DNA repair genes and markers for vascular stiffness, which is associated with aging. Our study supports the concept that genomic instability contributes importantly to the development of cardiovascular disease.
Conflict of interest statement
Figures



Similar articles
-
Phosphodiesterase 1 regulation is a key mechanism in vascular aging.Clin Sci (Lond). 2015 Dec;129(12):1061-75. doi: 10.1042/CS20140753. Epub 2015 Jun 25. Clin Sci (Lond). 2015. PMID: 26464516
-
Local endothelial DNA repair deficiency causes aging-resembling endothelial-specific dysfunction.Clin Sci (Lond). 2020 Apr 17;134(7):727-746. doi: 10.1042/CS20190124. Clin Sci (Lond). 2020. PMID: 32202295
-
Polymorphisms in ERCC1 and ERCC2/XPD genes and carcinogen DNA adducts in human lung.Lung Cancer. 2015 Jul;89(1):8-12. doi: 10.1016/j.lungcan.2015.05.001. Epub 2015 May 11. Lung Cancer. 2015. PMID: 26001533 Free PMC article.
-
Genetic polymorphisms in the nucleotide excision repair pathway and lung cancer risk: a meta-analysis.Int J Med Sci. 2007 Feb 1;4(2):59-71. doi: 10.7150/ijms.4.59. Int J Med Sci. 2007. PMID: 17299578 Free PMC article. Review.
-
Predictive Value of Ercc1 and Xpd Polymorphisms for Clinical Outcomes of Patients Receiving Neoadjuvant Therapy: A Prisma-Compliant Meta-Analysis.Medicine (Baltimore). 2015 Sep;94(39):e1593. doi: 10.1097/MD.0000000000001593. Medicine (Baltimore). 2015. PMID: 26426637 Free PMC article. Review.
Cited by
-
Role of Oxidative DNA Damage and Repair in Atrial Fibrillation and Ischemic Heart Disease.Int J Mol Sci. 2021 Apr 7;22(8):3838. doi: 10.3390/ijms22083838. Int J Mol Sci. 2021. PMID: 33917194 Free PMC article. Review.
-
Bad Smells and Broken DNA: A Tale of Sulfur-Nucleic Acid Cooperation.Antioxidants (Basel). 2021 Nov 17;10(11):1820. doi: 10.3390/antiox10111820. Antioxidants (Basel). 2021. PMID: 34829691 Free PMC article. Review.
-
Vascular Aging and Atherosclerosis: A Perspective on Aging.Aging Dis. 2024 Mar 6;16(1):33-48. doi: 10.14336/AD.2024.0201-1. Online ahead of print. Aging Dis. 2024. PMID: 38502584 Free PMC article.
-
Natural and non-natural antioxidative compounds: potential candidates for treatment of vascular calcification.Cell Death Discov. 2019 Nov 13;5:145. doi: 10.1038/s41420-019-0225-z. eCollection 2019. Cell Death Discov. 2019. PMID: 31754473 Free PMC article. Review.
-
Xeroderma Pigmentosum Group D (XPD) Inhibits the Proliferation Cycle of Vascular Smooth Muscle Cell (VSMC) by Activating Glycogen Synthase Kinase 3β (GSK3β).Med Sci Monit. 2018 Aug 27;24:5951-5959. doi: 10.12659/MSM.909614. Med Sci Monit. 2018. PMID: 30146633 Free PMC article.
References
-
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. - PubMed
-
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Fasolo CB, Sudano I, Salvetti A. Hypertension causes premature aging of endothelial function in humans. Hypertension. 1997;29:736–743. - PubMed
-
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. - PubMed
-
- Minamino T, Komuro I. Vascular cell senescence: contribution to atherosclerosis. Circ Res. 2007;100:15–26. - PubMed
-
- Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL071039/HL/NHLBI NIH HHS/United States
- N01 AG012100/AG/NIA NIH HHS/United States
- N01 AG062101/AG/NIA NIH HHS/United States
- R01 HL107385/HL/NHLBI NIH HHS/United States
- R01 HL076784/HL/NHLBI NIH HHS/United States
- R01 AG032098/AG/NIA NIH HHS/United States
- P30 DK072488/DK/NIDDK NIH HHS/United States
- N01 AG062103/AG/NIA NIH HHS/United States
- 233424/ERC_/European Research Council/International
- P01 AG017242/AG/NIA NIH HHS/United States
- 1P01 AG-17242-02/AG/NIA NIH HHS/United States
- R01 AG028321/AG/NIA NIH HHS/United States
- R01 HL070100/HL/NHLBI NIH HHS/United States
- R01 AG018728/AG/NIA NIH HHS/United States
- U01 ES011044/ES/NIEHS NIH HHS/United States
- K12 RR023250/RR/NCRR NIH HHS/United States
- R01 HL060040/HL/NHLBI NIH HHS/United States
- 1UO1 ES011044/ES/NIEHS NIH HHS/United States
- N01 AG062106/AG/NIA NIH HHS/United States
- K24 HL004334/HL/NHLBI NIH HHS/United States
- U01 HL084756/HL/NHLBI NIH HHS/United States
- R01 HL077447/HL/NHLBI NIH HHS/United States
- N01 AG012109/AG/NIA NIH HHS/United States
- U01 HL072515/HL/NHLBI NIH HHS/United States
- R01 HL080124/HL/NHLBI NIH HHS/United States
- R01 HL094898/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

