Glutathione redox potential in the mitochondrial intermembrane space is linked to the cytosol and impacts the Mia40 redox state
- PMID: 22705944
- PMCID: PMC3400016
- DOI: 10.1038/emboj.2012.165
Glutathione redox potential in the mitochondrial intermembrane space is linked to the cytosol and impacts the Mia40 redox state
Abstract
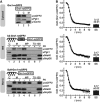
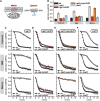
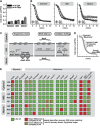
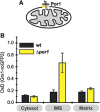
Glutathione is an important mediator and regulator of cellular redox processes. Detailed knowledge of local glutathione redox potential (E(GSH)) dynamics is critical to understand the network of redox processes and their influence on cellular function. Using dynamic oxidant recovery assays together with E(GSH)-specific fluorescent reporters, we investigate the glutathione pools of the cytosol, mitochondrial matrix and intermembrane space (IMS). We demonstrate that the glutathione pools of IMS and cytosol are dynamically interconnected via porins. In contrast, no appreciable communication was observed between the glutathione pools of the IMS and matrix. By modulating redox pathways in the cytosol and IMS, we find that the cytosolic glutathione reductase system is the major determinant of E(GSH) in the IMS, thus explaining a steady-state E(GSH) in the IMS which is similar to the cytosol. Moreover, we show that the local E(GSH) contributes to the partially reduced redox state of the IMS oxidoreductase Mia40 in vivo. Taken together, we provide a comprehensive mechanistic picture of the IMS redox milieu and define the redox influences on Mia40 in living cells.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Antos N, Budzinska M, Kmita H (2001) An interplay between the TOM complex and porin isoforms in the yeast Saccharomyces cerevisiae mitochondria. FEBS Lett 500: 12–16 - PubMed
-
- Appenzeller-Herzog C (2011) Glutathione- and non-glutathione-based oxidant control in the endoplasmic reticulum. J Cell Sci 124: 847–855 - PubMed
-
- Banhegyi G, Lusini L, Puskas F, Rossi R, Fulceri R, Braun L, Mile V, di Simplicio P, Mandl J, Benedetti A (1999) Preferential transport of glutathione versus glutathione disulfide in rat liver microsomal vesicles. J Biol Chem 274: 12213–12216 - PubMed
-
- Bien M, Longen S, Wagener N, Chwalla I, Herrmann JM, Riemer J (2010) Mitochondrial disulfide bond formation is driven by intersubunit electron transfer in Erv1 and proofread by glutathione. Mol Cell 37: 516–528 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

