Improvement in long-term outcomes with successive Total Therapy trials for multiple myeloma: are patients now being cured?
- PMID: 22705990
- PMCID: PMC3744094
- DOI: 10.1038/leu.2012.160
Improvement in long-term outcomes with successive Total Therapy trials for multiple myeloma: are patients now being cured?
Abstract
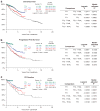
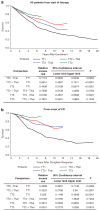
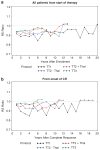
The concept of applying all active therapeutic agents in Total Therapy (TT) clinical trials for newly diagnosed multiple myeloma was pursued with the intent of developing curative treatment. The results of TT1 (n=231), TT2 (n=668) without or with thalidomide and TT3 with added bortezomib (n=303) have been reported. An update with median follow-up times of 17.1, 8.7 and 5.5 years, respectively, is provided. Conditional overall survival (OS) analysis from a 4-year landmark was applied to account for earlier protocol failure owing to disease aggressiveness and toxicities. Cumulative relative survival was computed in the context of age- and gender-matched US population, and interval-specific relative survival ratios were estimated to determine times to normal survival expectation. Based on Cox model-adjusted statistics, OS, progression-free survival and complete-response duration all improved with the transitions from TT1 to TT2 to TT3; improvement was also evident from time-to-progression estimates, 4-year conditional survival data and cumulative relative survival. Interval-specific relative survival normalized progressively sooner, reaching near-normal levels with TT3 in patients who attained complete response. Thus, a strategy using all myeloma-effective agents up-front seems effective at preventing, in progressively larger patient cohorts over time, the outgrowth of resistant tumor cells that account for ongoing relapses.
Conflict of interest statement
Dr Usmani is a consultant to Celgene, Millennium and Onyx and has received speaking honoraria from Celgene. Dr Barlogie has received research funding from Celgene and Novartis. He is a consultant to Celgene and Genzyme and has received speaking honoraria from Celgene and Millennium. Dr Barlogie is a co-inventor on patents and patent applications related to use of GEP in cancer medicine.
Figures


References
-
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–1060. - PubMed
-
- Barlogie B, Tricot GJ, van Rhee F, Angtuaco E, Walker R, Epstein J, et al. Long-term outcome results of the first tandem autotransplant trial for multiple myeloma. Br J Haematol. 2006;135:158–164. - PubMed
-
- Barlogie B, Tricot G, Anaissie E, Shaughnessy J, Rasmussen E, van Rhee F, et al. Thalidomide and hematopoietic-cell transplantation for multiple myeloma. N Engl J Med. 2006;354:1021–1030. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

