Surface display of N-terminally anchored invasin by Lactobacillus plantarum activates NF-κB in monocytes
- PMID: 22706054
- PMCID: PMC3406107
- DOI: 10.1128/AEM.01227-12
Surface display of N-terminally anchored invasin by Lactobacillus plantarum activates NF-κB in monocytes
Abstract
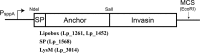
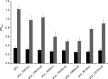
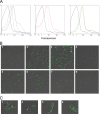
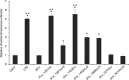
The probiotic lactic acid bacterium Lactobacillus plantarum is a potential delivery vehicle for mucosal vaccines because of its generally regarded as safe (GRAS) status and ability to persist at the mucosal surfaces of the human intestine. However, the inherent immunogenicity of vaccine antigens is in many cases insufficient to elicit an efficient immune response, implying that additional adjuvants are needed to enhance the antigen immunogenicity. The goal of the present study was to increase the proinflammatory properties of L. plantarum by expressing a long (D1 to D5 [D1-D5]) and a short (D4-D5) version of the extracellular domain of invasin from the human pathogen Yersinia pseudotuberculosis. To display these proteins on the bacterial surface, four different N-terminal anchoring motifs from L. plantarum were used, comprising two different lipoprotein anchors, a transmembrane signal peptide anchor, and a LysM-type anchor. All these anchors mediated surface display of invasin, and several of the engineered strains were potent activators of NF-κB when interacting with monocytes in cell culture. The most distinct NF-κB responses were obtained with constructs in which the complete invasin extracellular domain was fused to a lipoanchor. The proinflammatory L. plantarum strains constructed here represent promising mucosal delivery vehicles for vaccine antigens.
Figures
Similar articles
-
Immunogenic Properties of Lactobacillus plantarum Producing Surface-Displayed Mycobacterium tuberculosis Antigens.Appl Environ Microbiol. 2016 Dec 30;83(2):e02782-16. doi: 10.1128/AEM.02782-16. Print 2017 Jan 15. Appl Environ Microbiol. 2016. PMID: 27815271 Free PMC article.
-
Display of a β-mannanase and a chitosanase on the cell surface of Lactobacillus plantarum towards the development of whole-cell biocatalysts.Microb Cell Fact. 2016 Oct 4;15(1):169. doi: 10.1186/s12934-016-0570-z. Microb Cell Fact. 2016. PMID: 27716231 Free PMC article.
-
Recombinant invasive Lactobacillus plantarum expressing fibronectin binding protein A induce specific humoral immune response by stimulating differentiation of dendritic cells.Benef Microbes. 2019 May 28;10(5):589-604. doi: 10.3920/BM2018.0157. Epub 2019 May 15. Benef Microbes. 2019. PMID: 31088293
-
The benefits of Lactiplantibacillus plantarum: From immunomodulator to vaccine vector.Immunol Lett. 2025 Apr;272:106971. doi: 10.1016/j.imlet.2025.106971. Epub 2025 Jan 5. Immunol Lett. 2025. PMID: 39765312 Review.
-
Interaction of Yersinia enterocolitica with epithelial cells: invasin beyond invasion.Int J Med Microbiol. 2003 Apr;293(1):41-54. doi: 10.1078/1438-4221-00243. Int J Med Microbiol. 2003. PMID: 12755365 Review.
Cited by
-
Bioengineered probiotics, a strategic approach to control enteric infections.Bioengineered. 2013 Nov-Dec;4(6):379-87. doi: 10.4161/bioe.23574. Epub 2013 Jan 17. Bioengineered. 2013. PMID: 23327986 Free PMC article. Review.
-
Lactobacillus plantarum displaying CCL3 chemokine in fusion with HIV-1 Gag derived antigen causes increased recruitment of T cells.Microb Cell Fact. 2015 Oct 22;14:169. doi: 10.1186/s12934-015-0360-z. Microb Cell Fact. 2015. PMID: 26494531 Free PMC article.
-
Proof of concept in utilizing in-trans surface display system of Lactobacillus plantarum as mucosal tuberculosis vaccine via oral administration in mice.BMC Biotechnol. 2018 Oct 11;18(1):63. doi: 10.1186/s12896-018-0461-y. BMC Biotechnol. 2018. PMID: 30309359 Free PMC article.
-
Cell Wall Anchoring of a Bacterial Chitosanase in Lactobacillus plantarum Using a Food-Grade Expression System and Two Versions of an LP TG Anchor.Int J Mol Sci. 2020 May 27;21(11):3773. doi: 10.3390/ijms21113773. Int J Mol Sci. 2020. PMID: 32471049 Free PMC article.
-
Oral or intranasal immunization with recombinant Lactobacillus plantarum displaying head domain of Swine Influenza A virus hemagglutinin protects mice from H1N1 virus.Microb Cell Fact. 2022 Sep 9;21(1):185. doi: 10.1186/s12934-022-01911-4. Microb Cell Fact. 2022. PMID: 36085207 Free PMC article.
References
-
- Audouy SA, et al. 2007. Development of lactococcal GEM-based pneumococcal vaccines. Vaccine 25:2497–2506 - PubMed
-
- Azizi A, Kumar A, Diaz-Mitoma F, Mestecky J. 2010. Enhancing oral vaccine potency by targeting intestinal M cells. PLoS Pathog. 6:e1001147 doi:10.1371/journal.ppat.1001147 - DOI - PMC - PubMed
-
- Bermúdez-Humáran LG, et al. 2004. An inducible surface presentation system improves cellular immunity against human papillomavirus type 16 E7 antigen in mice after nasal administration with recombinant lactococci. J. Med. Microbiol. 53:427–433 - PubMed
-
- Bermúdez-Humáran LG, et al. 2005. A novel mucosal vaccine based on live lactococci expressing E7 antigen and IL-12 induces systemic and mucosal immune responses and protects mice against human papillomavirus type 16-induced tumors. J. Immunol. 175:7297–7302 - PubMed
-
- Bermúdez-Humáran LG, Kharrat P, Chatel JM, Langella P. 2011. Lactococci and lactobacilli as mucosal delivery vectors for therapeutic proteins and DNA vaccines. Microb. Cell Fact. 10(Suppl 1):S4 doi:10.1186/1475-2859-10-S1-S4 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

