Gemfibrozil, a lipid-lowering drug, upregulates IL-1 receptor antagonist in mouse cortical neurons: implications for neuronal self-defense
- PMID: 22706077
- PMCID: PMC3523125
- DOI: 10.4049/jimmunol.1102624
Gemfibrozil, a lipid-lowering drug, upregulates IL-1 receptor antagonist in mouse cortical neurons: implications for neuronal self-defense
Abstract
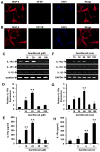
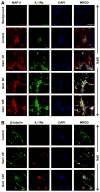
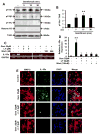
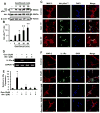
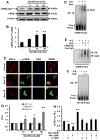
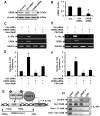
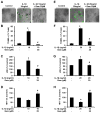
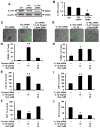
Chronic inflammation is becoming a hallmark of several neurodegenerative disorders and accordingly, IL-1β, a proinflammatory cytokine, is implicated in the pathogenesis of neurodegenerative diseases. Although IL-1β binds to its high-affinity receptor, IL-1R, and upregulates proinflammatory signaling pathways, IL-1R antagonist (IL-1Ra) adheres to the same receptor and inhibits proinflammatory cell signaling. Therefore, upregulation of IL-1Ra is considered important in attenuating inflammation. The present study underlines a novel application of gemfibrozil (gem), a Food and Drug Administration-approved lipid-lowering drug, in increasing the expression of IL-1Ra in primary mouse and human neurons. Gem alone induced an early and pronounced increase in the expression of IL-1Ra in primary mouse cortical neurons. Activation of type IA p110α PI3K and Akt by gem and abrogation of gem-induced upregulation of IL-1Ra by inhibitors of PI3K and Akt indicate a role of the PI3K-Akt pathway in the upregulation of IL-1Ra. Gem also induced the activation of CREB via the PI3K-Akt pathway, and small interfering RNA attenuation of CREB abolished the gem-mediated increase in IL-1Ra. Furthermore, gem was able to protect neurons from IL-1β insult. However, small interfering RNA knockdown of neuronal IL-1Ra abrogated the protective effect of gem against IL-1β, suggesting that this drug increases the defense mechanism of cortical neurons via upregulation of IL-1Ra. Taken together, these results highlight the importance of the PI3K-Akt-CREB pathway in mediating gem-induced upregulation of IL-1Ra in neurons and suggest gem as a possible therapeutic treatment for propagating neuronal self-defense in neuroinflammatory and neurodegenerative disorders.
Figures
References
-
- Alzheimer’s disease facts and figures. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2011;7:208–244. - PubMed
-
- Martin JB. Molecular basis of the neurodegenerative disorders. N Engl J Med. 1999;340:1970–1980. - PubMed
-
- Cummings JL, Vinters HV, Cole GM, Khachaturian ZS. Alzheimer’s disease: etiologies, pathophysiology, cognitive reserve, and treatment opportunities. Neurology. 1998;51:S2–17. discussion S65–17. - PubMed
-
- Griffin WS, Sheng JG, Roberts GW, Mrak RE. Interleukin-1 expression in different plaque types in Alzheimer’s disease: significance in plaque evolution. J Neuropathol Exp Neurol. 1995;54:276–281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

