CD2AP/SHIP1 complex positively regulates plasmacytoid dendritic cell receptor signaling by inhibiting the E3 ubiquitin ligase Cbl
- PMID: 22706086
- PMCID: PMC3665352
- DOI: 10.4049/jimmunol.1200887
CD2AP/SHIP1 complex positively regulates plasmacytoid dendritic cell receptor signaling by inhibiting the E3 ubiquitin ligase Cbl
Abstract
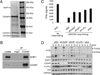
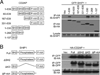
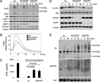
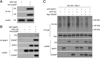
The human plasmacytoid dendritic cell (pDC) receptor BDCA2 forms a complex with the adaptor FcεR1γ to activate an ITAM-signaling cascade. BDCA2 receptor signaling negatively regulates the TLR7/9-mediated type 1 IFN responses in pDCs, which may play a key role in controlling self-DNA/RNA-induced autoimmunity. We report in this article that CD2-associated adaptor protein (CD2AP), which is highly expressed in human pDCs, positively regulates BDCA2/FcεR1γ receptor signaling. By immunoprecipitation and mass spectrometry analyses, we found that CD2AP bound to SHIP1. Knockdown of CD2AP or SHIP1 reduced the BDCA2/FcεR1γ-mediated ITAM signaling and blocked its inhibition of TLR9-mediated type 1 IFN production. Knockdown of CD2AP or SHIP1 also enhanced the ubiquitination and degradation of Syk and FcεR1γ that was mediated by the E3 ubiquitin ligase Cbl. This led us to discover that, upon BDCA2 cross-linking, the CD2AP/SHIP1 complex associated with Cbl and inhibited its E3 ubiquitin ligase activity. In human primary pDCs, cross-linking of the BDCA2/FcεR1γ complex induced the recruitment of the CD2AP/SHIP1/Cbl complex to the plasma membrane of pDCs, where it colocalized with the BDCA2/FcεR1γ complex. Therefore, CD2AP positively regulates BDCA2/FcεR1γ signaling by forming a complex with SHIP1 to inhibit the E3 ubiquitin ligase Cbl.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures


Similar articles
-
CD2-associated protein (CD2AP) enhances casitas B lineage lymphoma-3/c (Cbl-3/c)-mediated Ret isoform-specific ubiquitination and degradation via its amino-terminal Src homology 3 domains.J Biol Chem. 2014 Mar 14;289(11):7307-19. doi: 10.1074/jbc.M113.537878. Epub 2014 Jan 14. J Biol Chem. 2014. PMID: 24425877 Free PMC article.
-
TLR9/TLR7-triggered downregulation of BDCA2 expression on human plasmacytoid dendritic cells from healthy individuals and lupus patients.Clin Immunol. 2008 Oct;129(1):40-8. doi: 10.1016/j.clim.2008.06.004. Epub 2008 Aug 5. Clin Immunol. 2008. PMID: 18684674
-
CD2AP and Cbl-3/Cbl-c constitute a critical checkpoint in the regulation of ret signal transduction.J Neurosci. 2008 Aug 27;28(35):8789-800. doi: 10.1523/JNEUROSCI.2738-08.2008. J Neurosci. 2008. PMID: 18753381 Free PMC article.
-
Ubiquitin ligase Cbl-b and inhibitory Cblin peptides.Biochim Biophys Acta Proteins Proteom. 2020 Nov;1868(11):140495. doi: 10.1016/j.bbapap.2020.140495. Epub 2020 Jul 12. Biochim Biophys Acta Proteins Proteom. 2020. PMID: 32663526 Review.
-
SHIP1/2 interaction with tyrosine phosphorylated peptides mimicking an immunoreceptor signalling motif.Adv Enzyme Regul. 2006;46:142-53. doi: 10.1016/j.advenzreg.2006.01.013. Epub 2006 Jul 31. Adv Enzyme Regul. 2006. PMID: 16876851 Review. No abstract available.
Cited by
-
INPP5D rs35349669 polymorphism with late-onset Alzheimer's disease: A replication study and meta-analysis.Oncotarget. 2016 Oct 25;7(43):69225-69230. doi: 10.18632/oncotarget.12648. Oncotarget. 2016. PMID: 27750211 Free PMC article.
-
The MEK1/2-ERK Pathway Inhibits Type I IFN Production in Plasmacytoid Dendritic Cells.Front Immunol. 2018 Feb 26;9:364. doi: 10.3389/fimmu.2018.00364. eCollection 2018. Front Immunol. 2018. PMID: 29535732 Free PMC article.
-
Microglia in Alzheimer's disease.J Cell Biol. 2018 Feb 5;217(2):459-472. doi: 10.1083/jcb.201709069. Epub 2017 Dec 1. J Cell Biol. 2018. PMID: 29196460 Free PMC article. Review.
-
Targeted genetic analysis of cerebral blood flow imaging phenotypes implicates the INPP5D gene.Neurobiol Aging. 2019 Sep;81:213-221. doi: 10.1016/j.neurobiolaging.2019.06.003. Epub 2019 Jun 18. Neurobiol Aging. 2019. PMID: 31319229 Free PMC article.
-
Type I Interferon Production of Plasmacytoid Dendritic Cells under Control.Int J Mol Sci. 2021 Apr 18;22(8):4190. doi: 10.3390/ijms22084190. Int J Mol Sci. 2021. PMID: 33919546 Free PMC article. Review.
References
-
- Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 2005;23:275–306. - PubMed
-
- Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. - PubMed
-
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. - PubMed
-
- Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. - PubMed
-
- Barrat FJ, Meeker T, Chan JH, Guiducci C, Coffman RL. Treatment of lupus-prone mice with a dual inhibitor of TLR7 and TLR9 leads to reduction of autoantibody production and amelioration of disease symptoms. Eur. J. Immunol. 2007;37:3582–3586. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

