Distinct molecular pathways mediate glial activation and engulfment of axonal debris after axotomy
- PMID: 22706267
- PMCID: PMC4976689
- DOI: 10.1038/nn.3135
Distinct molecular pathways mediate glial activation and engulfment of axonal debris after axotomy
Abstract
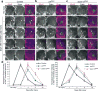
Glial cells efficiently recognize and clear cellular debris after nervous system injury to maintain brain homeostasis, but pathways governing glial responses to neural injury remain poorly defined. We identify the Drosophila melanogaster guanine nucleotide exchange factor complex Crk/Mbc/dCed-12 and the small GTPase Rac1 as modulators of glial clearance of axonal debris. We found that Crk/Mbc/dCed-12 and Rac1 functioned in a non-redundant fashion with the Draper transmembrane receptor pathway: loss of either pathway fully suppressed clearance of axonal debris. Draper signaling was required early during glial responses, promoting glial activation, which included increased Draper and dCed-6 expression and extension of glial membranes to degenerating axons. In contrast, the Crk/Mbc/dCed-12 complex functioned at later phases, promoting glial phagocytosis of axonal debris. Our work identifies new components of the glial engulfment machinery and shows that glial activation, phagocytosis of axonal debris and termination of responses to injury are genetically separable events mediated by distinct signaling pathways.
Figures



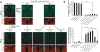



Similar articles
-
DRK/DOS/SOS converge with Crk/Mbc/dCed-12 to activate Rac1 during glial engulfment of axonal debris.Proc Natl Acad Sci U S A. 2014 Aug 26;111(34):12544-9. doi: 10.1073/pnas.1403450111. Epub 2014 Aug 6. Proc Natl Acad Sci U S A. 2014. PMID: 25099352 Free PMC article.
-
Negative regulation of glial engulfment activity by Draper terminates glial responses to axon injury.Nat Neurosci. 2012 Mar 18;15(5):722-30. doi: 10.1038/nn.3066. Nat Neurosci. 2012. PMID: 22426252 Free PMC article.
-
Astrocytes engage unique molecular programs to engulf pruned neuronal debris from distinct subsets of neurons.Genes Dev. 2014 Jan 1;28(1):20-33. doi: 10.1101/gad.229518.113. Epub 2013 Dec 20. Genes Dev. 2014. PMID: 24361692 Free PMC article.
-
Keeping the CNS clear: glial phagocytic functions in Drosophila.Glia. 2011 Sep;59(9):1304-11. doi: 10.1002/glia.21098. Epub 2010 Dec 6. Glia. 2011. PMID: 21136555 Review.
-
Central neuron-glial and glial-glial interactions following axon injury.Prog Neurobiol. 1998 May;55(1):1-26. doi: 10.1016/s0301-0082(97)00093-2. Prog Neurobiol. 1998. PMID: 9602498 Review.
Cited by
-
Prion-like transmission of neuronal huntingtin aggregates to phagocytic glia in the Drosophila brain.Nat Commun. 2015 Apr 13;6:6768. doi: 10.1038/ncomms7768. Nat Commun. 2015. PMID: 25866135 Free PMC article.
-
Downregulation of glial genes involved in synaptic function mitigates Huntington's disease pathogenesis.Elife. 2021 Apr 19;10:e64564. doi: 10.7554/eLife.64564. Elife. 2021. PMID: 33871358 Free PMC article.
-
Perineurial glia are essential for motor axon regrowth following nerve injury.J Neurosci. 2014 Sep 17;34(38):12762-77. doi: 10.1523/JNEUROSCI.1906-14.2014. J Neurosci. 2014. PMID: 25232113 Free PMC article.
-
Protein phosphatase 4 coordinates glial membrane recruitment and phagocytic clearance of degenerating axons in Drosophila.Cell Death Dis. 2017 Feb 23;8(2):e2623. doi: 10.1038/cddis.2017.40. Cell Death Dis. 2017. PMID: 28230857 Free PMC article.
-
Glial cells in neuronal development: recent advances and insights from Drosophila melanogaster.Neurosci Bull. 2014 Aug;30(4):584-94. doi: 10.1007/s12264-014-1448-2. Neurosci Bull. 2014. PMID: 25015062 Free PMC article. Review.
References
-
- Cuadros MA, Navascues J. The origin and differentiation of microglial cells during development. Prog Neurobiol. 1998;56:173–189. - PubMed
-
- Marin-Teva JL, Cuadros MA, Calvente R, Almendros A, Navascues J. Naturally occurring cell death and migration of microglial precursors in the quail retina during normal development. J Comp Neurol. 1999;412:255–275. - PubMed
-
- Parnaik R, Raff MC, Scholes J. Differences between the clearance of apoptotic cells by professional and non-professional phagocytes. Curr Biol. 2000;10:857–860. - PubMed
-
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

