Fungal antioxidant pathways promote survival against neutrophils during infection
- PMID: 22706306
- PMCID: PMC3534057
- DOI: 10.1172/JCI63239
Fungal antioxidant pathways promote survival against neutrophils during infection
Abstract
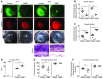
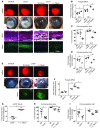
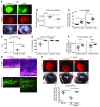
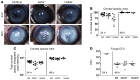
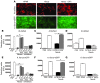
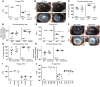
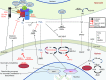
Filamentous fungi are a common cause of blindness and visual impairment worldwide. Using both murine model systems and in vitro human neutrophils, we found that NADPH oxidase produced by neutrophils was essential to control the growth of Aspergillus and Fusarium fungi in the cornea. We demonstrated that neutrophil oxidant production and antifungal activity are dependent on CD18, but not on the β-glucan receptor dectin-1. We used mutant A. fumigatus strains to show that the reactive oxygen species-sensing transcription factor Yap1, superoxide dismutases, and the Yap1-regulated thioredoxin antioxidant pathway are each required for protection against neutrophil-mediated oxidation of hyphae as well as optimal survival of fungal hyphae in vivo. We also demonstrated that thioredoxin inhibition using the anticancer drug PX-12 increased the sensitivity of fungal hyphae to both H2O2- and neutrophil-mediated killing in vitro. Additionally, topical application of PX-12 significantly enhanced neutrophil-mediated fungal killing in infected mouse corneas. Cumulatively, our data reveal critical host oxidative and fungal anti-oxidative mediators that regulate hyphal survival during infection. Further, these findings also indicate that targeting fungal anti-oxidative defenses via PX-12 may represent an efficacious strategy for treating fungal infections.
Figures



References
-
- Liesegang TJ, Forster RK. Spectrum of microbial keratitis in South Florida. Am J Ophthalmol. 1980;90(1):38–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

