The Ets transcription factor Spi-B is essential for the differentiation of intestinal microfold cells
- PMID: 22706340
- PMCID: PMC3704196
- DOI: 10.1038/ni.2352
The Ets transcription factor Spi-B is essential for the differentiation of intestinal microfold cells
Abstract
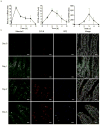
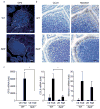
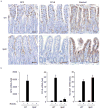
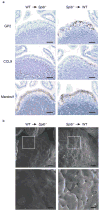
Intestinal microfold cells (M cells) are an enigmatic lineage of intestinal epithelial cells that initiate mucosal immune responses through the uptake and transcytosis of luminal antigens. The mechanisms of M-cell differentiation are poorly understood, as the rarity of these cells has hampered analysis. Exogenous administration of the cytokine RANKL can synchronously activate M-cell differentiation in mice. Here we show the Ets transcription factor Spi-B was induced early during M-cell differentiation. Absence of Spi-B silenced the expression of various M-cell markers and prevented the differentiation of M cells in mice. The activation of T cells via an oral route was substantially impaired in the intestine of Spi-B-deficient (Spib(-/-)) mice. Our study demonstrates that commitment to the intestinal M-cell lineage requires Spi-B as a candidate master regulator.
Figures



References
-
- Kraehenbuhl JP, Neutra MR. Epithelial M cells: differentiation and function. Annu Rev Cell Dev Biol. 2000;16:301–332. - PubMed
-
- Neutra MR, Mantis NJ, Kraehenbuhl JP. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat Immunol. 2001;2:1004–1009. - PubMed
-
- Neutra MR, Frey A, Kraehenbuhl JP. Epithelial M cells: gateways for mucosal infection and immunization. Cell. 1996;86:345–348. - PubMed
-
- Bockman DE, Cooper MD. Pinocytosis by epithelium associated with lymphoid follicles in the bursa of Fabricius, appendix, and Peyer’s patches. An electron microscopic study. Am J Anat. 1973;136:455–477. - PubMed
-
- Owen RL, Jones AL. Epithelial cell specialization within human Peyer’s patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology. 1974;66:189–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

