Pharmacokinetics of transdermal buprenorphine patch in the elderly
- PMID: 22706617
- PMCID: PMC3548110
- DOI: 10.1007/s00228-012-1320-8
Pharmacokinetics of transdermal buprenorphine patch in the elderly
Abstract
Purpose: Transdermal buprenorphine patches provide comparable pain relief to that of low-potency opioids in elderly individuals. However, specific data on their use in elderly individuals is limited. This study investigated and compared the PK of buprenorphine transdermal patches in elderly (≥ 75 years) versus younger (50-60 years) individuals.
Methods: This was a multiple-dose, open-label, parallel-group study in healthy volunteers split into two age groups (younger, 50-60 years; elderly, ≥ 75 years) with 37 individuals in each. Study participants received two consecutive 7-day buprenorphine 5 μg/h transdermal patch applications, and blood samples were collected on the week of the second patch application [day 7 (predose), days 8, 9, 10, 12, and 14] to determine PK at steady state. Pharmacokinetic parameters were determined for buprenorphine and norbuprenorphine. Safety was assessed by analyzing adverse events, hematology, clinical chemistry, urine analysis, vital signs, electrocardiogram (ECG), and physical examinations.
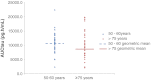
Results: The area under the plasma concentration-time curve at steady state (AUC(tau)), measured over one dosing interval, was similar for elderly [mean ± standard deviation (SD) 9,940 pg/h/ml (4,827 pg/h/ml] and younger [mean ± SD 11,309 (3,670 pg/h/ml] individuals. Bioequivalence was not demonstrated between groups, which may be attributable to the relatively high level of variability in individual plasma profiles. More adverse events were reported by younger (216) than elderly (164) study participants.
Conclusions: No dosage alterations are necessary for PK reasons when treating elderly people with buprenorphine transdermal patches.
Figures

Comment in
-
Transdermal buprenorphine-interesting observations on the metabolite norbuprenorphine levels in elderly subjects.Eur J Clin Pharmacol. 2013 Aug;69(8):1607-8. doi: 10.1007/s00228-013-1508-6. Epub 2013 Apr 16. Eur J Clin Pharmacol. 2013. PMID: 23588566 No abstract available.
-
Response to the letter to the editor by Dr. Srinivas on the recent publication of Al-Tawil et al. on the pharmacokinetics of transdermal buprenorphine patch in the elderly.Eur J Clin Pharmacol. 2013 Aug;69(8):1609-10. doi: 10.1007/s00228-013-1509-5. Epub 2013 Apr 16. Eur J Clin Pharmacol. 2013. PMID: 23588567 Free PMC article. No abstract available.
References
-
- Swedish Medical Products Agency. (2004) Treatment of osteoarthritis [in Swedish]. 13:19-25
-
- NICE Clinical Guideline for care and management in adults – Osteoarthritis (http://www.scribd.com/doc/2663331/NICE-guideline - accessed October 2011)
-
- American Geriatric Society (AGS) Panel on pharmacological management of persistent pain in older persons. J Am Geriatc Soc. 2009;67:1331–46. - PubMed
-
- The Swedish Council on Technology Assessment in Healthcare. (2009) Pharmaceutical treatments among the elderly – how can it be improved? [in Swedish]. Stockholm, Sweden: Swedish Council on Technology Assessment in Healthcare (www.sbu.se)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

