Targeting of the enhanced green fluorescent protein reporter to adrenergic cells in mice
- PMID: 22706789
- PMCID: PMC11104505
- DOI: 10.1007/s12033-012-9570-3
Targeting of the enhanced green fluorescent protein reporter to adrenergic cells in mice
Abstract
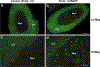
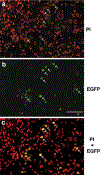
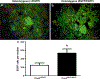
Adrenaline and noradrenaline are important neurotransmitter hormones that mediate physiological stress responses in adult mammals, and are essential for cardiovascular function during a critical period of embryonic/fetal development. In this study, we describe a novel mouse model system for identifying and characterizing adrenergic cells. Specifically, we generated a reporter mouse strain in which a nuclear-localized enhanced green fluorescent protein gene (nEGFP) was inserted into exon 1 of the gene encoding Phenylethanolamine n-methyltransferase (Pnmt), the enzyme responsible for production of adrenaline from noradrenaline. Our analysis demonstrates that this knock-in mutation effectively marks adrenergic cells in embryonic and adult mice. We see expression of nEGFP in Pnmt-expressing cells of the adrenal medulla in adult animals. We also note that nEGFP expression recapitulates the restricted expression of Pnmt in the embryonic heart. Finally, we show that nEGFP and Pnmt expressions are each induced in parallel during the in vitro differentiation of pluripotent mouse embryonic stem cells into beating cardiomyocytes. Thus, this new mouse genetic model should be useful for the identification and functional characterization of adrenergic cells in vitro and in vivo.
Figures





Similar articles
-
Visualization and ablation of phenylethanolamine N-methyltransferase producing cells in transgenic mice.Transgenic Res. 1994 Nov;3(6):388-400. doi: 10.1007/BF01976770. Transgenic Res. 1994. PMID: 8000434
-
Optogenetic Control of Heart Rhythm by Selective Stimulation of Cardiomyocytes Derived from Pnmt+ Cells in Murine Heart.Sci Rep. 2017 Jan 13;7:40687. doi: 10.1038/srep40687. Sci Rep. 2017. PMID: 28084430 Free PMC article.
-
Gene expression of phenylethanolamine N-methyltransferase in corticotropin-releasing hormone knockout mice during stress exposure.Cell Mol Neurobiol. 2006 Jul-Aug;26(4-6):735-54. doi: 10.1007/s10571-006-9063-7. Epub 2006 May 12. Cell Mol Neurobiol. 2006. PMID: 16691441 Free PMC article.
-
Why is the adrenal adrenergic?Endocr Pathol. 2003 Spring;14(1):25-36. doi: 10.1385/ep:14:1:25. Endocr Pathol. 2003. PMID: 12746560 Review.
-
Location, development, control, and function of extraadrenal phenylethanolamine N-methyltransferase.Ann N Y Acad Sci. 2002 Oct;971:76-82. doi: 10.1111/j.1749-6632.2002.tb04437.x. Ann N Y Acad Sci. 2002. PMID: 12438093 Review.
Cited by
-
Identification of the Sex of Pre-implantation Mouse Embryos Using a Marked Y Chromosome and CRISPR/Cas9.Sci Rep. 2019 Oct 4;9(1):14315. doi: 10.1038/s41598-019-50731-x. Sci Rep. 2019. PMID: 31586114 Free PMC article.
-
Metabolomics reveals critical adrenergic regulatory checkpoints in glycolysis and pentose-phosphate pathways in embryonic heart.J Biol Chem. 2018 May 4;293(18):6925-6941. doi: 10.1074/jbc.RA118.002566. Epub 2018 Mar 14. J Biol Chem. 2018. PMID: 29540484 Free PMC article.
-
Identification of the copy number variant biomarkers for breast cancer subtypes.Mol Genet Genomics. 2019 Feb;294(1):95-110. doi: 10.1007/s00438-018-1488-4. Epub 2018 Sep 10. Mol Genet Genomics. 2019. PMID: 30203254
-
Therapeutic potential of Pnmt+ primer cells for neuro/myocardial regeneration.Am J Stem Cells. 2013 Dec 22;2(3):137-54. Am J Stem Cells. 2013. PMID: 24396707 Free PMC article. Review.
References
-
- Thomas SA, Matsumoto AM, & Palmiter RD (1995). Noradrenaline is essential for mouse fetal development. Nature, 374, 643–646. - PubMed
-
- Zhou QY, Quaife CJ, & Palmiter RD (1995). Targeted disruption of the tyrosine hydroxylase gene reveals that catecholamines are required for mouse fetal development. Nature, 374, 640–643. - PubMed
-
- Kvetnansky R, Sabban EL, & Palkovits M (2009). Catecholaminergic systems in stress: Structural and molecular genetic approaches. Physiological Reviews, 89, 535–606. - PubMed
-
- Bao X, Lu CM, Liu F, Gu Y, Dalton ND, Zhu BQ, et al. (2007). Epinephrine is required for normal cardiovascular responses to stress in the phenylethanolamine N-methyltransferase knockout mouse. Circulation, 116, 1024–1031. - PubMed
-
- Ignarro LJ, & Shideman FE (1968). Appearance and concentrations of catecholamines and their biosynthesis in the embryonic and developing chick. Journal of Pharmacology and Experimental Therapeutics, 159, 38–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

