Laminin regulates postnatal oligodendrocyte production by promoting oligodendrocyte progenitor survival in the subventricular zone
- PMID: 22706957
- PMCID: PMC5679225
- DOI: 10.1002/glia.22365
Laminin regulates postnatal oligodendrocyte production by promoting oligodendrocyte progenitor survival in the subventricular zone
Abstract
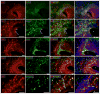
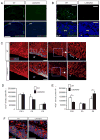
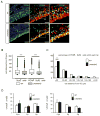
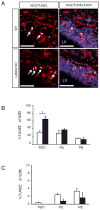
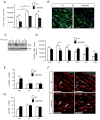
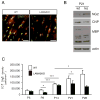
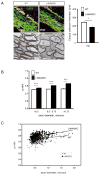
The laminin family of extracellular matrix proteins are expressed broadly during embryonic brain development, but are enriched at ventricular and pial surfaces where laminins mediate radial glial attachment during corticogenesis. In the adult brain, however, laminin distribution is restricted, yet is found within the vascular basal lamina and associated fractones of the ventricular zone (VZ)-subventricular zone (SVZ) stem cell niche, where laminins regulate adult neural progenitor cell proliferation. It remains unknown, however, if laminins regulate the wave of oligodendrogenesis that occurs in the neonatal/early postnatal VZ-SVZ. Here we report that Lama2, the gene that encodes the laminin α2-subunit, regulates postnatal oligodendrogenesis. At birth, Lama2-/- mice had significantly higher levels of dying oligodendrocyte progenitor cells (OPCs) in the OPC germinal zone of the dorsal SVZ. This translated into fewer OPCs, both in the dorsal SVZ well as in an adjacent developing white matter tract, the corpus callosum. In addition, intermediate progenitor cells that give rise to OPCs in the Lama2-/- VZ-SVZ were mislocalized and proliferated nearer to the ventricle surface. Later, delays in oligodendrocyte maturation (with accompanying OPC accumulation), were observed in the Lama2-/- corpus callosum, leading to dysmyelination by postnatal day 21. Together these data suggest that prosurvival laminin interactions in the developing postnatal VZ-SVZ germinal zone regulate the ability, or timing, of oligodendrocyte production to occur appropriately.
Copyright © 2012 Wiley Periodicals, Inc.
Figures

Similar articles
-
Neural and oligodendrocyte progenitor cells: transferrin effects on cell proliferation.ASN Neuro. 2013 Mar 8;5(1):e00107. doi: 10.1042/AN20120075. ASN Neuro. 2013. PMID: 23368675 Free PMC article.
-
Abundant occurrence of basal radial glia in the subventricular zone of embryonic neocortex of a lissencephalic primate, the common marmoset Callithrix jacchus.Cereb Cortex. 2012 Feb;22(2):469-81. doi: 10.1093/cercor/bhr301. Epub 2011 Nov 23. Cereb Cortex. 2012. PMID: 22114084 Free PMC article.
-
Oligodendrocyte lineage and subventricular zone response to traumatic axonal injury in the corpus callosum.J Neuropathol Exp Neurol. 2013 Dec;72(12):1106-25. doi: 10.1097/NEN.0000000000000009. J Neuropathol Exp Neurol. 2013. PMID: 24226267 Free PMC article.
-
Postnatal development of radial glia and the ventricular zone (VZ): a continuum of the neural stem cell compartment.Cereb Cortex. 2003 Jun;13(6):580-7. doi: 10.1093/cercor/13.6.580. Cereb Cortex. 2003. PMID: 12764031 Review.
-
Oligodendrogenesis in the subventricular zone and the role of epidermal growth factor.Brain Res Rev. 2011 Jun 24;67(1-2):147-56. doi: 10.1016/j.brainresrev.2011.01.001. Epub 2011 Jan 12. Brain Res Rev. 2011. PMID: 21236296 Free PMC article. Review.
Cited by
-
Laminin regulates oligodendrocyte development and myelination.Glia. 2022 Mar;70(3):414-429. doi: 10.1002/glia.24117. Epub 2021 Nov 12. Glia. 2022. PMID: 34773273 Free PMC article. Review.
-
Pathophysiology of the brain extracellular matrix: a new target for remyelination.Nat Rev Neurosci. 2013 Oct;14(10):722-9. doi: 10.1038/nrn3550. Epub 2013 Aug 29. Nat Rev Neurosci. 2013. PMID: 23985834 Review.
-
Mechanisms of oligodendrocyte regeneration from ventricular-subventricular zone-derived progenitor cells in white matter diseases.Front Cell Neurosci. 2013 Dec 26;7:275. doi: 10.3389/fncel.2013.00275. Front Cell Neurosci. 2013. PMID: 24421755 Free PMC article. Review.
-
Protection of Ndrg2 deficiency on renal ischemia-reperfusion injury via activating PINK1/Parkin-mediated mitophagy.Chin Med J (Engl). 2024 Nov 5;137(21):2603-2614. doi: 10.1097/CM9.0000000000002957. Epub 2024 Feb 26. Chin Med J (Engl). 2024. PMID: 38407220 Free PMC article.
-
Cottonseed oil alleviates ischemic stroke injury by inhibiting the inflammatory activation of microglia and astrocyte.J Neuroinflammation. 2020 Sep 11;17(1):270. doi: 10.1186/s12974-020-01946-7. J Neuroinflammation. 2020. PMID: 32917229 Free PMC article.
References
-
- Allamand V, Guicheney P. Merosin-deficient congenital muscular dystrophy, autosomal recessive (MDC1A, MIM# 156225, LAMA2 gene coding for α2 chain of laminin) European Journal of Human Genetics. 2002;10:91–94. - PubMed
-
- Benninger Y, Colognato H, Thurnherr T, Franklin RJM, Leone DP, Atanasoski S, Nave K-A, ffrench-Constant C, Suter U, Relvas JB. β1-integrin signaling mediates premyelinating oligodendrocyte survival but is not required for CNS myelination and remyelination. The Journal of Neuroscience. 2006;26:7665–7673. - PMC - PubMed
-
- Bongarzone ER. Induction of oligodendrocyte fate during the formation of the vertebrate neural tube. Neurochemical Research. 2002;27:1361–1369. - PubMed
-
- Buteică E, Roşulescu E, Burada F, Stănoiu B, Zăvăleanu M. Merosin-deficient congenital muscular dystrophy type 1A. Romanian Journal of Morphology and Embryology. 2008;49:229–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

