IL-29 is the dominant type III interferon produced by hepatocytes during acute hepatitis C virus infection
- PMID: 22706965
- PMCID: PMC3581145
- DOI: 10.1002/hep.25897
IL-29 is the dominant type III interferon produced by hepatocytes during acute hepatitis C virus infection
Abstract
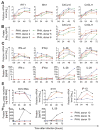
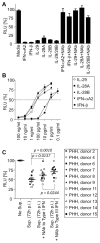
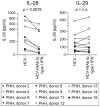
Early, vigorous intrahepatic induction of interferon (IFN)-stimulated gene (ISG) induction is a feature of hepatitis C virus (HCV) infection, even though HCV inhibits the induction of type I IFNs in vitro. To identify the cytokines and cells that drive ISG induction and mediate antiviral activity during acute HCV infection, type I and III IFN responses were studied in (1) serial liver biopsies and plasma samples obtained from 6 chimpanzees throughout acute HCV infection and (2) primary human hepatocyte (PHH) cultures upon HCV infection. Type I IFNs were minimally induced at the messenger RNA (mRNA) level in the liver and were undetectable at the protein level in plasma during acute HCV infection of chimpanzees. In contrast, type III IFNs, in particular, interleukin (IL)-29 mRNA and protein, were strongly induced and these levels correlated with ISG expression and viremia. However, there was no association between intrahepatic or peripheral type III IFN levels and the outcome of acute HCV infection. Infection of PHH with HCV recapitulated strong type III and weak type I IFN responses. Supernatants from HCV-infected PHH cultures mediated antiviral activity upon transfer to HCV-replicon-containing cells. This effect was significantly reduced by neutralization of type III IFNs and less by neutralization of type I IFNs. Furthermore, IL-29 production by HCV-infected PHH occurred independently from type I IFN signaling and was not enhanced by the presence of plasmacytoid dendritic cells.
Conclusion: Hepatocyte-derived type III IFNs contribute to ISG induction and antiviral activity, but are not the principal determinant of the outcome of HCV infection.
Copyright © 2012 American Association for the Study of Liver Diseases.
Conflict of interest statement
Potential conflict of interest: Nothing to report.
Figures




Similar articles
-
Independent, parallel pathways to CXCL10 induction in HCV-infected hepatocytes.J Hepatol. 2013 Oct;59(4):701-8. doi: 10.1016/j.jhep.2013.06.001. Epub 2013 Jun 12. J Hepatol. 2013. PMID: 23770038 Free PMC article.
-
HCV infection induces a unique hepatic innate immune response associated with robust production of type III interferons.Gastroenterology. 2012 Apr;142(4):978-88. doi: 10.1053/j.gastro.2011.12.055. Epub 2012 Jan 13. Gastroenterology. 2012. PMID: 22248663 Free PMC article.
-
Retinoic acid inducible gene-I (RIG-I) signaling of hepatic stellate cells inhibits hepatitis C virus replication in hepatocytes.Innate Immun. 2013;19(2):193-202. doi: 10.1177/1753425912460414. Epub 2012 Oct 11. Innate Immun. 2013. PMID: 23060457 Free PMC article.
-
Multi-step regulation of interferon induction by hepatitis C virus.Arch Immunol Ther Exp (Warsz). 2013 Apr;61(2):127-38. doi: 10.1007/s00005-012-0214-x. Epub 2013 Jan 5. Arch Immunol Ther Exp (Warsz). 2013. PMID: 23292079 Review.
-
Innate immunity and HCV.J Hepatol. 2013 Mar;58(3):564-74. doi: 10.1016/j.jhep.2012.10.005. Epub 2012 Oct 11. J Hepatol. 2013. PMID: 23063572 Review.
Cited by
-
Activation of Type I and Type III Interferons in Chronic Hepatitis C.J Innate Immun. 2015;7(3):251-259. doi: 10.1159/000369973. Epub 2015 Jan 28. J Innate Immun. 2015. PMID: 25766746 Free PMC article. Review.
-
Combinations of lambda interferon with direct-acting antiviral agents are highly efficient in suppressing hepatitis C virus replication.Antimicrob Agents Chemother. 2013 Mar;57(3):1312-22. doi: 10.1128/AAC.02239-12. Epub 2012 Dec 28. Antimicrob Agents Chemother. 2013. PMID: 23274666 Free PMC article.
-
Basal interferon signaling and therapeutic use of interferons in controlling rotavirus infection in human intestinal cells and organoids.Sci Rep. 2018 May 29;8(1):8341. doi: 10.1038/s41598-018-26784-9. Sci Rep. 2018. PMID: 29844362 Free PMC article.
-
Innate immunity in stem cell-derived hepatocytes.Philos Trans R Soc Lond B Biol Sci. 2018 Jul 5;373(1750):20170220. doi: 10.1098/rstb.2017.0220. Philos Trans R Soc Lond B Biol Sci. 2018. PMID: 29786555 Free PMC article. Review.
-
IFN-λ3 polymorphism indirectly influences NK cell phenotype and function during acute HCV infection.Immun Inflamm Dis. 2016 Aug 16;4(3):376-88. doi: 10.1002/iid3.122. eCollection 2016 Sep. Immun Inflamm Dis. 2016. PMID: 27621819 Free PMC article.
References
-
- Ciesek S, Manns MP. Hepatitis in 2010: the dawn of a new era in HCV therapy. Nat Rev Gastroenterol Hepatol. 2011;8:69–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
