Ultrastructural remodelling of Ca(2+) signalling apparatus in failing heart cells
- PMID: 22707157
- PMCID: PMC3422078
- DOI: 10.1093/cvr/cvs195
Ultrastructural remodelling of Ca(2+) signalling apparatus in failing heart cells
Abstract
Aims: The contraction of a heart cell is controlled by Ca(2+)-induced Ca(2+) release between L-type Ca(2+) channels (LCCs) in the cell membrane/T-tubules (TTs) and ryanodine receptors (RyRs) in the junctional sarcoplasmic reticulum (SR). During heart failure, LCC-RyR signalling becomes defective. The purpose of the present study was to reveal the ultrastructural mechanism underlying the defective LCC-RyR signalling and contractility.
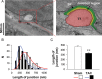
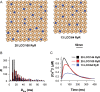
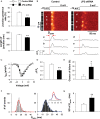
Methods and results: In rat models of heart failure produced by transverse aortic constriction surgery, stereological analysis of transmission electron microscopic images showed that the volume density and the surface area of junctional SRs and those of SR-coupled TTs were both decreased in failing heart cells. The TT-SR junctions were displaced or missing from the Z-line areas. Moreover, the spatial span of individual TT-SR junctions was markedly reduced in failing heart cells. Numerical simulation and junctophilin-2 knockdown experiments demonstrated that the decrease in junction size (and thereby the constitutive LCC and RyR numbers) led to a scattered delay of Ca(2+) release activation.
Conclusions: The shrinking and eventual absence of TT-SR junctions are important mechanisms underlying the desynchronized and inhomogeneous Ca(2+) release and the decreased contractile strength in heart failure. Maintaining the nanoscopic integrity of TT-SR junctions thus represents a therapeutic strategy against heart failure and related cardiomyopathies.
Figures



Comment in
-
Calcium release units in heart failure: that's about the size of it.Cardiovasc Res. 2012 Sep 1;95(4):397-8. doi: 10.1093/cvr/cvs241. Epub 2012 Jul 24. Cardiovasc Res. 2012. PMID: 22832099 No abstract available.
References
-
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. - PubMed
-
- Lopez-Lopez JR, Shacklock PS, Balke CW, Wier WG. Local calcium transients triggered by single L-type calcium channel currents in cardiac cells. Science. 1995;268:1042–1045. - PubMed
-
- Rios E, Stern MD. Calcium in close quarters: microdomain feedback in excitation-contraction coupling and other cell biological phenomena. Annu Rev Biophys Biomol Struct. 1997;26:47–82. - PubMed
-
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

