Trimethylamine N-oxide as a media supplement for cartilage tissue engineering
- PMID: 22707357
- PMCID: PMC3625430
- DOI: 10.1002/jor.22171
Trimethylamine N-oxide as a media supplement for cartilage tissue engineering
Abstract
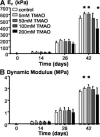
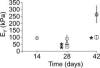
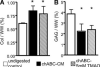
Supplements added to the culture media (e.g., growth factors and dexamethasone) have been successful in improving mechanical and biochemical properties of engineered cartilage towards native values. Trimethylamine N-oxide (TMAO), a natural osmolyte found in shark cartilage, is thought to induce protein folding, and counteracts the destabilizing effect of the high concentrations of urea stored by sharks. The objective of this study was to investigate the use of TMAO as a media supplement for promoting growth of functional engineered cartilage in culture. In the first study, TMAO was added to the culture media for the first 14 days in culture and concentrations of 0-200 mM were evaluated. In the second study, TMAO was supplemented to the culture media following chondroitinase ABC digestion, which has been previously shown to mediate an increased collagen content in engineered cartilage. A dose-dependent response was observed with improved mechanical and biochemical properties for engineered constructs cultured with TMAO at concentrations of 5-100 mM. The Young's modulus of digested constructs cultured in TMAO was 2× greater than digested constructs cultured in the control medium and recovered to undigested control levels by day 42. In conclusion, these initial studies with TMAO as a media supplement show promise for improving the compressive mechanical properties, increasing extracellular matrix production, and increasing the recovery time following chABC digestion.
Copyright © 2012 Orthopaedic Research Society.
Figures





References
-
- Guilak F, Sah RL, Setton LA. Physical regulation of cartilage metabolism. In: Mov VC, Hayes WC, editors. Basic orthopaedic biomechanics. Raven Press; New York: 1997. pp. 197–207.
-
- Mow VC, Bachrach NM, Setton LA, et al. Stress, strain, pressure, and flow fields in articular cartilage and chondrocytes. In: Mov VC, Guilak F, Hayes WC, editors. Cell mechanics and cellular engineering. Springer; New York: 1994. pp. 345–379.
-
- Kuo CK, Li WJ, Mauck RL, et al. Cartilage tissue engineering: its potential and uses. Curr Opin Rheumatol. 2006;18:64–73. - PubMed
-
- Toh WS, Lee EH, Cao T. Potential of human embryonic stem cells in cartilage tissue engineering and regenerative medicine. Stem Cell Rev. 2011;7:544–559. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

