Efficacy, safety, and biomarkers of single-agent bevacizumab therapy in patients with advanced hepatocellular carcinoma
- PMID: 22707516
- PMCID: PMC3425524
- DOI: 10.1634/theoncologist.2011-0465
Efficacy, safety, and biomarkers of single-agent bevacizumab therapy in patients with advanced hepatocellular carcinoma
Abstract
Objective: Hepatocellular carcinoma (HCC) is a highly vascularized tumor in which neoangiogenesis contributes to growth and metastasis. We assessed the safety, efficacy, and potential biomarkers of activity of bevacizumab in patients with advanced HCC.
Methods: In this phase II trial, eligible patients received bevacizumab, 5 mg/kg or 10 mg/kg every 2 weeks. The disease-control rate at 16 weeks (16W-DCR) was the primary endpoint. Circulating endothelial cells (CECs) and plasma cytokines and angiogenic factors (CAFs) were measured at baseline and throughout treatment.
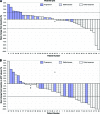
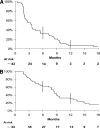
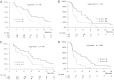
Results: The 16W-DCR was 42% (95% confidence interval, 27%-57%). Six of the 43 patients who received bevacizumab achieved a partial response (objective response rate [ORR], 14%). Grade 3-4 asthenia, hemorrhage, and aminotransferase elevation occurred in five (12%), three (7%), and three (7%) patients, respectively. During treatment, placental growth factor markedly increased, whereas vascular endothelial growth factor (VEGF)-A dramatically decreased (p < .0001); soluble VEGF receptor-2 (p < .0001) and CECs (p = .03) transiently increased on day 3. High and increased CEC counts at day 15 were associated with the ORR (p = .04) and the 16W-DCR (p = .02), respectively. Lower interleukin (IL)-8 levels at baseline (p = .01) and throughout treatment (p ≤ .04) were associated with the 16W-DCR. High baseline IL-8 and IL-6 levels predicted shorter progression-free and overall survival times (p ≤ .04).
Conclusion: Bevacizumab is active and well tolerated in patients with advanced HCC. The clinical value of CECs, IL-6, and IL-8 warrants further investigation.
Trial registration: ClinicalTrials.gov NCT00162669.
Conflict of interest statement
Figures
References
-
- Ferlay J, Autier P, Boniol M, et al. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–592. - PubMed
-
- Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. - PubMed
-
- Lopez PM, Villanueva A, Llovet JM. Systematic review: Evidence-based management of hepatocellular carcinoma—an updated analysis of randomized controlled trials. Aliment Pharmacol Ther. 2006;23:1535–1547. - PubMed
-
- Pang R, Poon RT. Angiogenesis and antiangiogenic therapy in hepatocellular carcinoma. Cancer Lett. 2006;242:151–167. - PubMed
-
- Tseng PL, Tai MH, Huang CC, et al. Overexpression of VEGF is associated with positive p53 immunostaining in hepatocellular carcinoma (HCC) and adverse outcome of HCC patients. J Surg Oncol. 2008;98:349–357. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

