Lethal, hereditary mutants of phospholamban elude phosphorylation by protein kinase A
- PMID: 22707725
- PMCID: PMC3411000
- DOI: 10.1074/jbc.M112.382713
Lethal, hereditary mutants of phospholamban elude phosphorylation by protein kinase A
Abstract
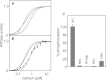
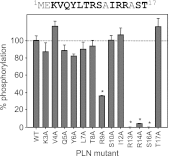
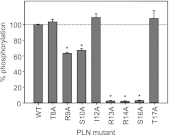
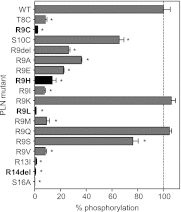
The sarcoplasmic reticulum calcium pump (SERCA) and its regulator, phospholamban, are essential components of cardiac contractility. Phospholamban modulates contractility by inhibiting SERCA, and this process is dynamically regulated by β-adrenergic stimulation and phosphorylation of phospholamban. Herein we reveal mechanistic insight into how four hereditary mutants of phospholamban, Arg(9) to Cys, Arg(9) to Leu, Arg(9) to His, and Arg(14) deletion, alter regulation of SERCA. Deletion of Arg(14) disrupts the protein kinase A recognition motif, which abrogates phospholamban phosphorylation and results in constitutive SERCA inhibition. Mutation of Arg(9) causes more complex changes in function, where hydrophobic substitutions such as cysteine and leucine eliminate both SERCA inhibition and phospholamban phosphorylation, whereas an aromatic substitution such as histidine selectively disrupts phosphorylation. We demonstrate that the role of Arg(9) in phospholamban function is multifaceted: it is important for inhibition of SERCA, it increases the efficiency of phosphorylation, and it is critical for protein kinase A recognition in the context of the phospholamban pentamer. Given the synergistic consequences on contractility, it is not surprising that the mutants cause lethal, hereditary dilated cardiomyopathy.
Figures
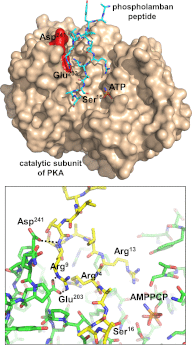
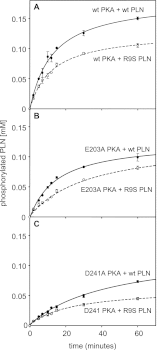
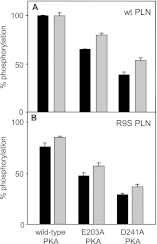
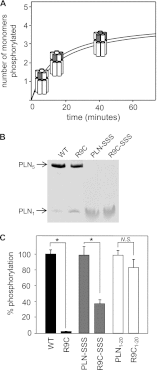
References
-
- Tada M., Kirchberger M. A., Katz A. M. (1976) Regulation of calcium transport in cardiac sarcoplasmic reticulum by cyclic AMP-dependent protein kinase. Recent Adv. Stud. Cardiac Struct. Metab. 9, 225–239 - PubMed
-
- Morgan J. P. (1991) Abnormal intracellular modulation of calcium as a major cause of cardiac contractile dysfunction. N. Engl. J. Med. 325, 625–632 - PubMed
-
- Simmerman H. K., Collins J. H., Theibert J. L., Wegener A. D., Jones L. R. (1986) Sequence analysis of phospholamban. Identification of phosphorylation sites and two major structural domains. J. Biol. Chem. 261, 13333–13341 - PubMed
-
- Schmidt A. G., Edes I., Kranias E. G. (2001) Phospholamban. A promising therapeutic target in heart failure? Cardiovasc. Drugs Ther. 15, 387–396 - PubMed
-
- Grünig E., Tasman J. A., Kücherer H., Franz W., Kübler W., Katus H. A. (1998) Frequency and phenotypes of familial dilated cardiomyopathy. J. Am. Coll. Cardiol. 31, 186–194 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

