A bipartite autoinhibitory region within the B-domain suppresses function in factor V
- PMID: 22707727
- PMCID: PMC3406718
- DOI: 10.1074/jbc.M112.377168
A bipartite autoinhibitory region within the B-domain suppresses function in factor V
Abstract
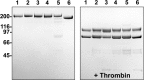
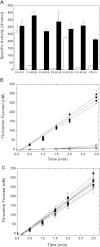
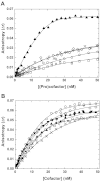
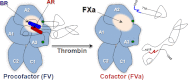
Activation of blood coagulation factor V (FV) is a key reaction of hemostasis. FV circulates in plasma as an inactive procofactor, and proteolytic removal of a large central B-domain converts it to an active cofactor (FVa) for factor Xa (FXa). Here we show that two short evolutionary conserved segments of the B-domain, together termed the procofactor regulatory region, serve an essential autoinhibitory function. This newly identified motif consists of a basic (963-1008) and an acidic (1493-1537) region and defines the minimal sequence requirements to maintain FV as a procofactor. Our data suggest that dismantling this autoinhibitory region via deletion or proteolysis is the driving force to unveil a high affinity binding site(s) for FXa. These findings document an unexpected sequence-specific role for the B-domain by negatively regulating FV function and preventing activity of the procofactor. These new mechanistic insights point to new ways in which the FV procofactor to cofactor transition could be modulated to alter hemostasis.
Figures



References
-
- Mann K. G., Kalafatis M. (2003) Factor V. A combination of Dr. Jekyll and Mr. Hyde. Blood 101, 20–30 - PubMed
-
- Mann K. G., Nesheim M. E., Church W. R., Haley P., Krishnaswamy S. (1990) Surface-dependent reactions of the vitamin K-dependent enzyme complexes. Blood 76, 1–16 - PubMed
-
- Nesheim M. E., Mann K. G. (1979) Thrombin-catalyzed activation of single chain bovine factor V. J. Biol. Chem. 254, 1326–1334 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

