Polymeric conjugates for drug delivery
- PMID: 22707853
- PMCID: PMC3374380
- DOI: 10.1021/cm2031569
Polymeric conjugates for drug delivery
Abstract
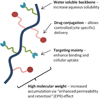
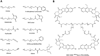
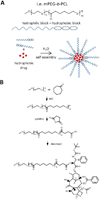
The field of polymer therapeutics has evolved over the past decade and has resulted in the development of polymer-drug conjugates with a wide variety of architectures and chemical properties. Whereas traditional non-degradable polymeric carriers such as poly(ethylene glycol) (PEG) and N-(2-hydroxypropyl methacrylamide) (HPMA) copolymers have been translated to use in the clinic, functionalized polymer-drug conjugates are increasingly being utilized to obtain biodegradable, stimuli-sensitive, and targeted systems in an attempt to further enhance localized drug delivery and ease of elimination. In addition, the study of conjugates bearing both therapeutic and diagnostic agents has resulted in multifunctional carriers with the potential to both "see and treat" patients. In this paper, the rational design of polymer-drug conjugates will be discussed followed by a review of different classes of conjugates currently under investigation. The design and chemistry used for the synthesis of various conjugates will be presented with additional comments on their potential applications and current developmental status.
Figures



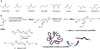

References
-
- Shelanski HA, Shelanski MV. PVP-iodine: history, toxicity and therapeutic uses. J Int Coll Surg. 1956;25(6):727–734. - PubMed
-
- Ravin HA, Seligman AM, Fine J. Polyvinyl pyrrolidone as a plasma expander. New Eng J Med. 1952;247(24):921–929. - PubMed
-
- Ringsdorf H. Structure and properties of pharmacologically active polymers. Journal of Polymer Science: Polymer Symposia. 1975;51(1):135–153.
-
- Zhou P, Li Z, Chau Y. Synthesis, characterization, and in vivo evaluation of poly(ethylene oxide-co-glycidol)-platinate conjugate. Eur J Pharm Sci. 2010;41(3–4):464–472. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
