Internal Spin Trapping of Thiyl Radical during the Complexation and Reduction of Cobalamin with Glutathione and Dithiothrietol
- PMID: 22707875
- PMCID: PMC3375740
- DOI: 10.1142/S1088424611004051
Internal Spin Trapping of Thiyl Radical during the Complexation and Reduction of Cobalamin with Glutathione and Dithiothrietol
Abstract
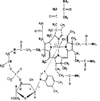
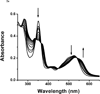
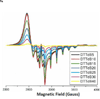
The activation of cobalamin requires the reduction of Cbl(III) to Cbl(II). The reduction by glutathione and dithiothreitol was followed using visible spectroscopy and electron paramagnetic resonance. In addition the oxidation of glutathione was monitored. Glutathione first reacts with oxidized Cbl(III). The binding of a second glutathione required for the reduction to Cbl(II) is presumably located in the dimethyl benzimidazole ribonucleotide ligand cavity. The reduction of Cbl(III) by dithiothreitol, which contains two thiols, is much faster even though no stable Cbl(III) complex is formed. The reduction, by both thiol reagents, results in the formation of thiyl radicals, some of which are released to form oxidized thiol products and some of which remain associated with the reduced cobalamin. In the reduced state the intrinsic lower affinity for the benzimidazole base, coupled with a trans effect from the initial GSH bound to the β-axial site and a possible lowering of the pH results in an equilibrium between base-on and base-off complexes. The dissociation of the base facilitates a closer approach of the thiyl radical to the Co(II) α-axial site resulting in a complex with ferromagnetic exchange coupling between the metal ion and the thiyl radical. This is a unique example of 'internal spin trapping' of a thiyl radical formed during reduction. The finding that the reduction involves a peripheral site and that thiyl radicals produced during the reduction remain associated with the reduced cobalamin provide important new insights into our understanding of the formation and function of cobalamin enzymes.
Figures
















Similar articles
-
Characterization of sulfur-centered radical intermediates formed during the oxidation of thiols and sulfite by peroxynitrite. ESR-spin trapping and oxygen uptake studies.J Biol Chem. 1996 Mar 15;271(11):6000-9. doi: 10.1074/jbc.271.11.6000. J Biol Chem. 1996. PMID: 8626383
-
Hypochlorite-induced oxidation of thiols: formation of thiyl radicals and the role of sulfenyl chlorides as intermediates.Free Radic Res. 2000 Dec;33(6):719-29. doi: 10.1080/10715760000301241. Free Radic Res. 2000. PMID: 11237094
-
Aniline-, phenylhydroxylamine-, nitrosobenzene-, and nitrobenzene-induced hemoglobin thiyl free radical formation in vivo and in vitro.Mol Pharmacol. 1990 Feb;37(2):311-8. Mol Pharmacol. 1990. PMID: 2154677
-
Thiyl radicals in biological systems: significant or trivial?Biochem Soc Symp. 1995;61:55-63. doi: 10.1042/bss0610055. Biochem Soc Symp. 1995. PMID: 8660403 Review.
-
Mechanisms of radical formation from reactions of ozone with target molecules in the lung.Free Radic Biol Med. 1994 Nov;17(5):451-65. doi: 10.1016/0891-5849(94)90172-4. Free Radic Biol Med. 1994. PMID: 7835752 Review.
Cited by
-
Pathogenic mutations differentially affect the catalytic activities of the human B12-processing chaperone CblC and increase futile redox cycling.J Biol Chem. 2015 May 1;290(18):11393-402. doi: 10.1074/jbc.M115.637132. Epub 2015 Mar 25. J Biol Chem. 2015. PMID: 25809485 Free PMC article.
-
Vitamin B12b Enhances the Cytotoxicity of Diethyldithiocarbamate in a Synergistic Manner, Inducing the Paraptosis-Like Death of Human Larynx Carcinoma Cells.Biomolecules. 2020 Jan 1;10(1):69. doi: 10.3390/biom10010069. Biomolecules. 2020. PMID: 31906414 Free PMC article.
-
Differences in the Formation of Reactive Oxygen Species and Their Cytotoxicity between Thiols Combined with Aqua- and Cyanocobalamins.Int J Mol Sci. 2022 Sep 20;23(19):11032. doi: 10.3390/ijms231911032. Int J Mol Sci. 2022. PMID: 36232333 Free PMC article.
-
Characterization of the complex between native and reduced bovine serum albumin with aquacobalamin and evidence of dual tetrapyrrole binding.J Biol Inorg Chem. 2018 Jul;23(5):725-738. doi: 10.1007/s00775-018-1562-8. Epub 2018 May 2. J Biol Inorg Chem. 2018. PMID: 29721769
-
Solid-state Porphyrin Interactions with Oppositely Charged Peripheral Groups.J Porphyr Phthalocyanines. 2015 Dec;19(12):1256-1261. doi: 10.1142/S1088424615501084. J Porphyr Phthalocyanines. 2015. PMID: 27547029 Free PMC article.
References
-
- McCaddon A, Regland B, Hudson P, Davies D. Neurology. 2002;58:1395–1399. - PubMed
-
- Kelly PJ, Rosand J, Kistler JP, Shih VE, Silveira S, Plomaritoglou A, Furie KL. Neurology. 2002;59:529–536. - PubMed
-
- Miyao Y, Fujimoto K, Kugiyama K, Kawano H, Hirai N, Sugiyama S, Sakamoto T, Yoshimura M, Ogawa H. Thrombosis Research. 2003;112:123–129. - PubMed
-
- Graham M, O’Callghan P. Cardiovascular drugs and therapy. 2002;16:383–389. - PubMed
-
- Wang FC, De Pasqua V, Gérard P, Delwaide PJ. Neurology. 2001;57:897–899. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
