Versatile Genetic Tool Box for the Crenarchaeote Sulfolobus acidocaldarius
- PMID: 22707949
- PMCID: PMC3374326
- DOI: 10.3389/fmicb.2012.00214
Versatile Genetic Tool Box for the Crenarchaeote Sulfolobus acidocaldarius
Abstract
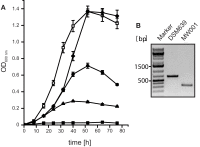
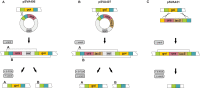
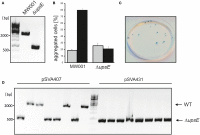
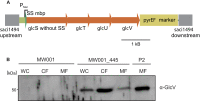
For reverse genetic approaches inactivation or selective modification of genes are required to elucidate their putative function. Sulfolobus acidocaldarius is a thermoacidophilic Crenarchaeon which grows optimally at 76°C and pH 3. As many antibiotics do not withstand these conditions the development of a genetic system in this organism is dependent on auxotrophies. Therefore we constructed a pyrE deletion mutant of S. acidocaldarius wild type strain DSM639 missing 322 bp called MW001. Using this strain as the starting point, we describe here different methods using single as well as double crossover events to obtain markerless deletion mutants, tag genes genomically and ectopically integrate foreign DNA into MW001. These methods enable us to construct single, double, and triple deletions strains that can still be complemented with the pRN1 based expression vector. Taken together we have developed a versatile and robust genetic tool box for the crenarchaeote S. acidocaldarius that will promote the study of unknown gene functions in this organism and makes it a suitable host for synthetic biology approaches.
Keywords: Sulfolobus; archaea; deletion mutant; expression system; genetics; in-frame deletion.
Figures


Similar articles
-
Disruption of the gene encoding restriction endonuclease SuaI and development of a host-vector system for the thermoacidophilic archaeon Sulfolobus acidocaldarius.Extremophiles. 2016 Mar;20(2):139-48. doi: 10.1007/s00792-016-0807-0. Epub 2016 Jan 20. Extremophiles. 2016. PMID: 26791382
-
Methods for Markerless Gene Deletion and Plasmid-Based Expression in Sulfolobus acidocaldarius.Methods Mol Biol. 2022;2522:135-144. doi: 10.1007/978-1-0716-2445-6_8. Methods Mol Biol. 2022. PMID: 36125747
-
The genome of Sulfolobus acidocaldarius, a model organism of the Crenarchaeota.J Bacteriol. 2005 Jul;187(14):4992-9. doi: 10.1128/JB.187.14.4992-4999.2005. J Bacteriol. 2005. PMID: 15995215 Free PMC article.
-
Homologous recombination in Sulfolobus acidocaldarius: genetic assays and functional properties.Biochem Soc Trans. 2009 Feb;37(Pt 1):88-91. doi: 10.1042/BST0370088. Biochem Soc Trans. 2009. PMID: 19143608 Review.
-
General vectors for archaeal hyperthermophiles: strategies based on a mobile intron and a plasmid.FEMS Microbiol Rev. 1996 May;18(2-3):93-104. doi: 10.1111/j.1574-6976.1996.tb00229.x. FEMS Microbiol Rev. 1996. PMID: 8639332 Review.
Cited by
-
Biosynthesis of GMGT lipids by a radical SAM enzyme associated with anaerobic archaea and oxygen-deficient environments.Nat Commun. 2024 Jun 19;15(1):5256. doi: 10.1038/s41467-024-49650-x. Nat Commun. 2024. PMID: 38898040 Free PMC article.
-
Sulfolobus acidocaldarius Transports Pentoses via a Carbohydrate Uptake Transporter 2 (CUT2)-Type ABC Transporter and Metabolizes Them through the Aldolase-Independent Weimberg Pathway.Appl Environ Microbiol. 2018 Jan 17;84(3):e01273-17. doi: 10.1128/AEM.01273-17. Print 2018 Feb 1. Appl Environ Microbiol. 2018. PMID: 29150511 Free PMC article.
-
Guide-independent DNA cleavage by archaeal Argonaute from Methanocaldococcus jannaschii.Nat Microbiol. 2017 Mar 20;2:17034. doi: 10.1038/nmicrobiol.2017.34. Nat Microbiol. 2017. PMID: 28319081 Free PMC article.
-
Gene deletions leading to a reduction in the number of cyclopentane rings in Sulfolobus acidocaldarius tetraether lipids.FEMS Microbiol Lett. 2018 Jan 1;365(1):fnx250. doi: 10.1093/femsle/fnx250. FEMS Microbiol Lett. 2018. PMID: 29211845 Free PMC article.
-
Metabolic engineering of Caldicellulosiruptor bescii for hydrogen production.Appl Microbiol Biotechnol. 2024 Dec;108(1):65. doi: 10.1007/s00253-023-12974-7. Epub 2024 Jan 9. Appl Microbiol Biotechnol. 2024. PMID: 38194138 Free PMC article. Review.
References
-
- Albers S. V., Birkeland N. K., Driessen A. J., Gertig S., Haferkamp P., Klenk H. P., Kouril T., Manica A., Pham T. K., Ruoff P., Schleper C., Schomburg D., Sharkey K. J., Siebers B., Sierocinski P., Steuer R., van der Oost J., Westerhoff H. V., Wieloch P., Wright P. C., Zaparty M. (2009). SulfoSYS (Sulfolobus Systems Biology): towards a silicon cell model for the central carbohydrate metabolism of the archaeon Sulfolobus solfataricus under temperature variation. Biochem. Soc. Trans. 37, 58–6410.1042/BST0370058 - DOI - PubMed
-
- Albers S. V., Jonuscheit M., Dinkelaker S., Urich T., Kletzin A., Tampe R., Driessen A. J. M., Schleper C. (2006). Production of recombinant and tagged proteins in the hyperthermophilic archaeon Sulfolobus solfataricus. Appl. Environ. Microbiol. 72, 102–11110.1128/AEM.72.1.102-111.2006 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

