Enhanced genotoxicity of silver nanoparticles in DNA repair deficient Mammalian cells
- PMID: 22707954
- PMCID: PMC3374476
- DOI: 10.3389/fgene.2012.00104
Enhanced genotoxicity of silver nanoparticles in DNA repair deficient Mammalian cells
Abstract
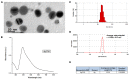
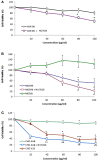
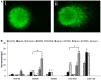
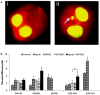
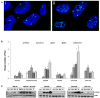
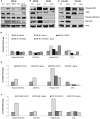
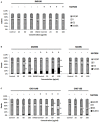
Silver nanoparticles (Ag-np) have been used in medicine and commercially due to their anti-microbial properties. Therapeutic potentials of these nanoparticles are being explored extensively despite the lack of information on their mechanism of action at molecular and cellular level. Here, we have investigated the DNA damage response and repair following Ag-np treatment in mammalian cells. Studies have shown that Ag-np exerts genotoxicity through double-strand breaks (DSBs). DNA-PKcs, the catalytic subunit of DNA dependent protein kinase, is an important caretaker of the genome which is known to be the main player mediating Non-homologous End-Joining (NHEJ) repair pathway. We hypothesize that DNA-PKcs is responsible for the repair of Ag-np induced DNA damage. In vitro studies have been carried out to investigate both cytotoxicity and genotoxicity induced by Ag-np in normal human cells, DNA-PKcs proficient, and deficient mammalian cells. Chemical inhibition of DNA-PKcs activity with NU7026, an ATP-competitive inhibitor of DNA-PKcs, has been performed to further validate the role of DNA-PKcs in this model. Our results suggest that Ag-np induced more prominent dose-dependent decrease in cell viability in DNA-PKcs deficient or inhibited cells. The deficiency or inhibition of DNA-PKcs renders the cells with higher susceptibility to DNA damage and genome instability which in turn contributed to greater cell cycle arrest/cell death. These findings support the fact that DNA-PKcs is involved in the repair of Ag-np induced genotoxicity and NHEJ repair pathway and DNA-PKcs particularly is activated to safeguard the genome upon Ag-np exposure.
Keywords: DNA damage and repair; DNA-PKcs; genotoxicity; silver nanoparticles.
Figures
Similar articles
-
DNA-dependent protein kinase modulates the anti-cancer properties of silver nanoparticles in human cancer cells.Mutat Res Genet Toxicol Environ Mutagen. 2017 Dec;824:32-41. doi: 10.1016/j.mrgentox.2017.10.001. Epub 2017 Oct 13. Mutat Res Genet Toxicol Environ Mutagen. 2017. PMID: 29150048
-
Biochemical evidence for Ku-independent backup pathways of NHEJ.Nucleic Acids Res. 2003 Sep 15;31(18):5377-88. doi: 10.1093/nar/gkg728. Nucleic Acids Res. 2003. PMID: 12954774 Free PMC article.
-
Extensive ssDNA end formation at DNA double-strand breaks in non-homologous end-joining deficient cells during the S phase.BMC Mol Biol. 2007 Oct 26;8:97. doi: 10.1186/1471-2199-8-97. BMC Mol Biol. 2007. PMID: 17963495 Free PMC article.
-
Mechanisms of DNA double strand break repair and chromosome aberration formation.Cytogenet Genome Res. 2004;104(1-4):14-20. doi: 10.1159/000077461. Cytogenet Genome Res. 2004. PMID: 15162010 Review.
-
DNA-PKcs: A Multi-Faceted Player in DNA Damage Response.Front Genet. 2020 Dec 23;11:607428. doi: 10.3389/fgene.2020.607428. eCollection 2020. Front Genet. 2020. PMID: 33424929 Free PMC article. Review.
Cited by
-
Cancer cells that survive checkpoint adaptation contain micronuclei that harbor damaged DNA.Cell Cycle. 2016 Nov 16;15(22):3131-3145. doi: 10.1080/15384101.2016.1231287. Epub 2016 Sep 16. Cell Cycle. 2016. PMID: 27636097 Free PMC article.
-
Heterogeneous Responses of Ovarian Cancer Cells to Silver Nanoparticles as a Single Agent and in Combination with Cisplatin.J Nanomater. 2017;2017:5107485. doi: 10.1155/2017/5107485. Epub 2017 Apr 26. J Nanomater. 2017. PMID: 30034459 Free PMC article.
-
Nanoscale wide-band semiconductors for photocatalytic remediation of aquatic pollution.Environ Sci Pollut Res Int. 2017 Nov;24(33):25775-25797. doi: 10.1007/s11356-017-0252-3. Epub 2017 Oct 7. Environ Sci Pollut Res Int. 2017. PMID: 28988306 Review.
-
Emerging metrology for high-throughput nanomaterial genotoxicology.Mutagenesis. 2017 Jan;32(1):215-232. doi: 10.1093/mutage/gew037. Epub 2016 Aug 26. Mutagenesis. 2017. PMID: 27565834 Free PMC article. Review.
-
The mechanism of cell death induced by silver nanoparticles is distinct from silver cations.Part Fibre Toxicol. 2021 Oct 14;18(1):37. doi: 10.1186/s12989-021-00430-1. Part Fibre Toxicol. 2021. PMID: 34649580 Free PMC article.
References
-
- An J., Huang Y. C., Xu Q. Z., Zhou L. J., Shang Z. F., Huang B., Wang Y., Liu X. D., Wu D. C., Zhou P. K. (2010). DNA-PKcs plays a dominant role in the regulation of H2AX phosphorylation in response to DNA damage and cell cycle progression. BMC Mol. Biol. 11, 18 10.1186/1471-2199-11-18 - DOI - PMC - PubMed
-
- Asharani P., Sethu S., Lim H. K., Balaji G., Valiyaveettil S., Hande M. P. (2012). Differential regulation of intracellular factors mediating cell cycle, DNA repair and inflammation following exposure to silver nanoparticles in human cells. Genome Integr. 3, 2.10.1186/2041-9414-3-2 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

