Incorporation of DOPE into Lipoplexes formed from a Ferrocenyl Lipid leads to Inverse Hexagonal Nanostructures that allow Redox-Based Control of Transfection in High Serum
- PMID: 22707977
- PMCID: PMC3374640
- DOI: 10.1039/C2SM00047D
Incorporation of DOPE into Lipoplexes formed from a Ferrocenyl Lipid leads to Inverse Hexagonal Nanostructures that allow Redox-Based Control of Transfection in High Serum
Abstract
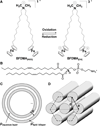
We report small angle X-ray and neutron scattering measurements that reveal that mixtures of the redox-active lipid bis(11-ferrocenylundecyl)dimethylammonium bromide (BFDMA) and dioleoylphosphatidylethanolamine (DOPE) spontaneously form lipoplexes with DNA that exhibit inverse hexagonal nanostructure (H(II) (c)). In contrast to lipoplexes of DNA and BFDMA only, which exhibit a multilamellar nanostructure (L(α) (c)) and limited ability to transfect cells in the presence of serum proteins, we measured lipoplexes of BFDMA and DOPE with the H(II) (c) nanostructure to survive incubation in serum and to expand significantly the range of media compositions (e.g., up to 80% serum) over which BFDMA can be used to transfect cells with high efficiency. Importantly, we also measured the oxidation state of the ferrocene within the BFDMA/DNA lipoplexes to have a substantial influence on the transfection efficiency of the lipoplexes in media containing serum. Specifically, whereas lipoplexes of reduced BFDMA and DOPE transfect cells with high efficiency, lipoplexes of oxidized BFDMA and DNA lead to low levels of transfection. Complementary measurements using SAXS reveal that the low transfection efficiency of the lipoplexes of oxidized BFDMA and DOPE correlates with the presence of weak Bragg peaks and thus low levels of H(II) (c) nanostructure in solution. Overall, these results provide support for our hypothesis that DOPE-induced formation of the H(II) (c) nanostructure of the BFDMA-containing lipoplexes underlies the high cell transfection efficiency measured in the presence of serum, and that the oxidation state of BFDMA within lipoplexes with DOPE substantially regulates the formation of the H(II) (c) nanostructure and thus the ability of the lipoplexes to transfect cells with DNA. More generally, the results presented in this paper suggest that lipoplexes formed from BFDMA and DOPE may offer the basis of approaches that permit active and external control of transfection of cells in the presence of high (physiologically relevant) levels of serum.
Figures


References
-
- Patnaik S, Tripathi SK, Goyal R, Arora A, Mitra K, Villaverde A, Vazquez E, Shukla Y, Kumar P, Gupta KC. Soft Matter. 2011;7:6103–6112.
-
- Ziauddin J, Sabatini DM. Nature. 2001;411:107–110. - PubMed
-
- Bailey SN, Wu RZ, Sabatini DM. Drug Discovery Today. 2002;7:S113–S118. - PubMed
-
- Delehanty JB, Shaffer KM, Lin BC. Anal. Chem. 2004;76:7323–7328. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

