Cardiac myosin binding protein-C: redefining its structure and function
- PMID: 22707987
- PMCID: PMC3374655
- DOI: 10.1007/s12551-012-0067-x
Cardiac myosin binding protein-C: redefining its structure and function
Abstract
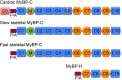
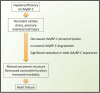
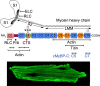
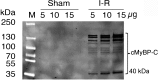
Mutations of cardiac myosin binding protein-C (cMyBP-C) are inherited by an estimated 60 million people worldwide, and the protein is the target of several kinases. Recent evidence further suggests that cMyBP-C mutations alter Ca(2+) transients, leading to electrophysiological dysfunction. Thus, while the importance of studying this cardiac sarcomere protein is clear, preliminary data in the literature have raised many questions. Therefore, in this article, we propose to review the structure and function of cMyBP-C with particular respect to the role(s) in cardiac contractility and whether its release into the circulatory system is a potential biomarker of myocardial infarction. We also discuss future directions and experimental designs that may lead to expanding the role(s) of cMyBP-C in the heart. In conclusion, we suggest that cMyBP-C is a regulatory protein that could offer a broad clinical utility in maintaining normal cardiac function.
Figures

References
-
- Ababou A, Gautel M, Pfuhl M. Dissecting the N-terminal myosin binding site of human cardiac myosin-binding protein C. Structure and myosin binding of domain C2. J Biol Chem. 2007;282(12):9204–9215. - PubMed
-
- Ahmad F, Banerjee SK, Lage ML, Huang XN, Smith SH, Saba S, Rager J, Conner DA, Janczewski AM, Tobita K, Tinney JP, Moskowitz IP, Perez-Atayde AR, Keller BB, Mathier MA, Shroff SG, Seidman CE, Seidman JG. The role of cardiac troponin T quantity and function in cardiac development and dilated cardiomyopathy. PLoS One. 2008;3:e2642. doi: 10.1371/journal.pone.0002642. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

