Heparin reduces neuroinflammation and transsynaptic neuronal apoptosis in a model of subarachnoid hemorrhage
- PMID: 22707992
- PMCID: PMC3372778
- DOI: 10.1007/s12975-012-0166-9
Heparin reduces neuroinflammation and transsynaptic neuronal apoptosis in a model of subarachnoid hemorrhage
Abstract
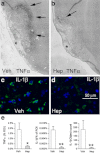
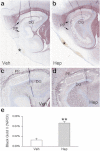
Subarachnoid hemorrhage (SAH) can lead to disabling motor, cognitive, and neuropsychological abnormalities. Part of the secondary injury to cerebral tissues associated with SAH is attributable to the neuroinflammatory response induced by blood. Heparin is a pleiotropic compound that reduces inflammatory responses in conditions outside the central nervous system. Using a model of SAH devoid of global insult, we evaluated the effect of delayed intravenous (IV) infusion of heparin, at a dose that does not produce therapeutic anticoagulation, on neuroinflammation, myelin preservation, and apoptosis. Adult male rats underwent bilateral stereotactic injections of autologous blood (50 μL) into the subarachnoid space of the entorhinal cortex. The rats were implanted with mini-osmotic pumps that delivered either vehicle or unfractionated heparin (10 U/kg/h IV) beginning 12 h after SAH. No mechanical or hemorrhagic injury was observed in the hippocampus. In vehicle controls assessed at 48 h, SAH was associated with robust neuroinflammation in the adjacent cortex [neutrophils, activated phagocytic microglia, nuclear factor-kappa B, tumor necrosis factor-alpha, and interleukin-1beta] and neurodegeneration (Fluoro-Jade C staining and loss of NeuN). In the hippocampus, a muted neuroinflammatory response was indicated by Iba1-positive, ED1-negative microglia exhibiting an activated morphology. The perforant pathway showed Fluoro-Jade C staining and demyelination, and granule cells of the dentate gyrus had pyknotic nuclei, labeled with Fluoro-Jade C and showed upregulation of cleaved caspase-3, consistent with transsynaptic apoptosis. Administration of heparin significantly reduced neuroinflammation, demyelination, and transsynaptic apoptosis. We conclude that delayed IV infusion of low-dose unfractionated heparin may attenuate adverse neuroinflammatory effects of SAH.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

