Optimizing the Temporal Resolution of Fast-Scan Cyclic Voltammetry
- PMID: 22708011
- PMCID: PMC3375060
- DOI: 10.1021/cn200119u
Optimizing the Temporal Resolution of Fast-Scan Cyclic Voltammetry
Abstract
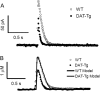
Electrochemical detection with carbon-fiber microelectrodes has become an established method to monitor directly the release of dopamine from neurons and its uptake by the dopamine transporter. With constant potential amperometry (CPA) the measured current provides a real time view of the rapid concentration changes, but the method lacks chemical identification of the monitored species and markedly increases the difficulty of signal calibration. Monitoring with fast-scan cyclic voltammetry (FSCV) allows species identification and concentration measurements, but often exhibits a delayed response time due to the time-dependent adsorption/desorption of electroactive species at the electrode. We sought to improve the temporal resolution of FSCV to make it more comparable to CPA by increasing the waveform repetition rate from 10 to 60 Hz with uncoated carbon-fiber electrodes. The faster acquisition led to diminished time delays of the recordings that tracked more closely with CPA measurements. The measurements reveal that FSCV at 10 Hz underestimates the normal rate of dopamine uptake by about 18%. However, FSCV collection at 10 Hz and 60 Hz provide identical results when a dopamine transporter (DAT) blocker such as cocaine is bath applied. To verify further the utility of this method, we used transgenic mice that over-express DAT. After accounting for the slight adsorption delay time, FSCV at 60 Hz adequately monitored the increased uptake rate that arose from overexpression of DAT and, again, was similar to CPA results. Furthermore, the utility of collecting data at 60 Hz was verified in an anesthetized rat by using a higher scan rate (2400 V/s) to increase sensitivity and the overall signal.
Figures




References
-
- Sulzer D.; Pothos E. N. (2000) Regulation of quantal size by presynaptic mechanisms. Rev. Neurosci. 11(2–3), 159–212. - PubMed
-
- Latini S.; Pedata F. (2001) Adenosine in the central nervous system: Release mechanisms and extracellular concentrations. J. Neurochem. 79(3), 463–484. - PubMed
-
- Giros B.; Jaber M.; Jones S. R.; Wightman R. M.; Caron M. G. (1996) Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 379, 606–612. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

