Development and Characterization of a Vaginal Film Containing Dapivirine, a Non- nucleoside Reverse Transcriptase Inhibitor (NNRTI), for prevention of HIV-1 sexual transmission
- PMID: 22708075
- PMCID: PMC3375737
- DOI: 10.1007/s13346-011-0022-6
Development and Characterization of a Vaginal Film Containing Dapivirine, a Non- nucleoside Reverse Transcriptase Inhibitor (NNRTI), for prevention of HIV-1 sexual transmission
Abstract
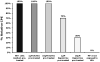
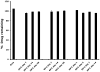
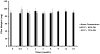
Dapivirine, a non-nucleoside reverse transcriptase inhibitor, is a potent and promising anti-HIV molecule. It is currently being investigated for use as a vaginal microbicide in two dosage forms, a semi-solid gel and a silicone elastomer ring. Quick-dissolving films are promising and attractive dosage forms that may provide an alternative platform for the vaginal delivery of microbicide drug candidates. Vaginal films may provide advantages such as discreet use, no product leakage during use, lack of requirement for an applicator for insertion, rapid drug release and minimal packaging and reduced wastage. Within this study the in vitro bioactivity of dapivirine as compared to the NNRTI UC781 was further established and a quick dissolve film was developed for vaginal application of dapivirine for prevention of HIV infection. The developed film was characterized with respect to its physical and chemical attributes including water content, mechanical strength, drug release profile, permeability, compatibility with lactobacilli and bioactivity. The anti-HIV activity of the formulated dapivirine film was confirmed in in vitro and ex vivo models. Importantly the physical and chemical properties of the film as well as its bioactivity were maintained for a period of 18 months. In conclusion, a vaginal film containing dapivirine was developed and characterized. The film was shown to prevent HIV-1 infection in vitro and ex vivo and have acceptable characteristics which make this film a promising candidate for testing as vaginal microbicide.
Figures







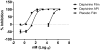

References
-
- UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2010. UNAIDS; 2010.
-
- Garg AB, Nuttall J, Romano J. The future of HIV microbicides: challenges and opportunities. Antivir Chem Chemother. 2009;19(4):143–50. - PubMed
-
- Zydowsky TM. Microbicides: chemistry, structure, and strategy. Curr Opin HIV AIDS. 2008 Sep;3(5):548–53. - PubMed
-
- Balzarini J, Van Damme L. Microbicide drug candidates to prevent HIV infection. Lancet. 2007 Mar 3;369(9563):787–97. - PubMed
-
- Hillier SL, Moench T, Shattock R, Black R, Reichelderfer P, Veronese F. In vitro and in vivo: the story of nonoxynol 9. J Acquir Immune Defic Syndr. 2005 May 1;39(1):1–8. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

