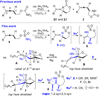
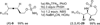Modular functionalization of allenes to aminated stereotriads
- PMID: 22708990
- PMCID: PMC4476975
- DOI: 10.1021/ja304859w
Modular functionalization of allenes to aminated stereotriads
Abstract
Nitrogen-containing stereotriads, compounds with three adjacent stereodefined carbons, are commonly found in biologically important molecules. However, the preparation of molecules bearing these motifs can be challenging. Herein, we describe a modular oxidation protocol which converts a substituted allene to a triply functionalized amine of the form C-X/C-N/C-Y. The key step employs a Rh-catalyzed intramolecular conversion of the allene to a strained bicyclic methylene aziridine. This reactive intermediate is further elaborated to the target products, often in one reaction vessel and with effective transfer of the axial chirality of the allene to point chirality in the stereotriad.
Figures
References
-
- Pasco M, Moumne R, Lecourt T, Micouin L. J. Org. Chem. 2011;76:5137. - PubMed
- King SB, Ganem B. J. Am. Chem. Soc. 1994;116:562.
- Magerlein BJ, Kagan F. J. Med. Chem. 1969;12:780. - PubMed
- Zhang Y, Liu S, Che Y, Liu X. J. Nat. Prod. 2007;70:1522. - PubMed
- Lewis JG, Anandan SK, O’dowd H, Gordeev MF. PCT Int. Appl. 2005 WO 2005012320 A2 20050210.
-
-
For selected examples of allene bis-epoxidation and subsequent transformations, see: Crandall JK, Batal DJ, Sebesta DP, Lin F. J. Org. Chem. 1991;56:1153. and references therein. Crandall JK, Batal DJ. J. Org. Chem. 1988;53:1338. and references therein. Yue Z, Williams LJ. J. Org. Chem. 2009;74:7707. Shangguan N, Kiren S, Williams LJ. Org. Lett. 2007;9:1093. Ghosh P, Cusick JR, Inghrim J, Williams LJ. Org. Lett. 2009;11:4672. Lotesta SD, Hou YQ, Williams LJ. Org. Lett. 2007;9:869. Joyasawal S, Lotesta SD, Akhmedov NG, Williams LJ. Org. Lett. 2010;12:988. Crandall JK, Batal DJ, Sebesta DP, Lin F. J. Org. Chem. 1991;56:1153. Liu K, Kim H, Ghosh P, Akhmedov NG, Williams LJ. J. Am. Chem. Soc. 2011;133:14968. Sharma R, Manpadi M, Zhang Y, Kim H, Ahmedov NG, Williams LJ. Org. Lett. 2011;13:3352. Ghosh P, Zhang Y, Emge TJ, Williams LJ. Org. Lett. 2009;11:4402. Kim H, Williams LJ. Curr. Opin. Drug Discovery Dev. 2008;11:870. Lotesta SD, Kiren S, Sauers RR, Williams LJ. Angew. Chem. Int. Ed. 2007;46:15. Ghosh P, Lotesta SD, Williams LJ. J. Am. Chem. Soc. 2007;129:2438. Katukojvala S, Barlett KN, Lotesta SD, Williams LJ. J. Am. Chem. Soc. 2004;126:15348.
-
-
- Robertson J, Feast GC, White LV, Steadman VA, Claridge TDW. Org. Biomol. Chem. 2010;8:3060. - PubMed
- Feast GC, Page LW, Robertson J. Chem. Commun. 2010;46:2835. - PubMed
- Grigg RD, Schomaker JM, Timokhin V. Tetrahedron. 2011;67:4318.
- Boralsky LA, Marston D, Grigg RD, Hershberger JC, Schomaker JM. Org. Lett. 2011;13:1924. - PMC - PubMed
- Rigoli JW, Boralsky LA, Hershberger JC, Marston D, Meis AR, Guzei IA, Schomaker JM. J. Org. Chem. 2012;77:2446. - PMC - PubMed
- Weatherly CD, Rigoli JW, Schomaker JM. Org. Lett. 2012;14:1704. - PMC - PubMed
- Stoll AH, Blakey SB. J. Am. Chem. Soc. 2010;132:2108. - PubMed
- Atkinson RS, Malpass JR. Tetrahedron Lett. 1975;48:4305.
-
-
For references on the synthesis and chemistry of non-bicyclic methylene aziridines, see: Tehrani KA, Kimpe N. Curr. Org. Chem. 2009;13:854. Shipman M. Synlett. 2006;19:3205. and references therein. Cariou CCA, Clarkson GJ, Shipman M. J. Org. Chem. 2008;73:9762.
-
-
-
Stereochemistry of Organic Compounds Eliel EL, Wilen SH. New York: John Wiley & Sons; 1994. pp. 696–697.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources