Human glioma cells induce hyperexcitability in cortical networks
- PMID: 22709330
- PMCID: PMC3418468
- DOI: 10.1111/j.1528-1167.2012.03557.x
Human glioma cells induce hyperexcitability in cortical networks
Abstract
Purpose: Patients with gliomas frequently present with seizures, but the factors associated with seizure development are still poorly understood. In this study, we assessed peritumoral synaptic network activity in a glioma animal model and tested the contribution of aberrant glutamate release from gliomas on glioma-associated epileptic network activity.
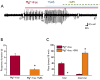
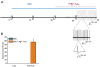
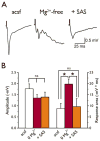
Methods: In vitro brain slices were made from glioma-implanted mice. Using extracellular field recordings, we analyzed peritumoral epileptiform activity induced by Mg(2+)-free medium in slices from tumor-bearing animals and sham-operated controls. We assessed the effect of sulfasalazine (SAS), a blocker of system and glutamate release, on spontaneous and evoked activity in tumor-associated slices.
Key findings: Tumor-associated cortical networks were hyperexcitable. The onset latency of Mg(2+)-free-induced epileptiform activity was significantly shorter in tumor-bearing slices, and the incidence of Mg(2+)-free-induced ictal-like events was higher. Block of glutamate release from system decreased the response area of evoked activity and completely blocked Mg(2+)-free-induced ictal-like, but not interictal-like events.
Significance: Control of seizures in patients with gliomas is an essential component of clinical management; therefore, understanding the origin of seizures is vital. This work provides evidence that peritumoral synaptic network activity is disrupted by tumor masses resulting in network excitability. We show that blocking glutamate release via system with SAS, a drug already approved by the U.S. Food and Drug Administration (FDA), can inhibit Mg(2+)-free-induced ictal-like epileptiform events similar to other chemicals used to decrease seizure activity. We, therefore, suggest that further studies should consider SAS a promising agent to aid in the treatment of seizures associated with gliomas.
Wiley Periodicals, Inc. © 2012 International League Against Epilepsy.
Conflict of interest statement
Disclosure: The authors report no conflicts of interest.
Figures



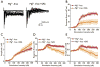
References
-
- Anderson MF, Blomstrand F, Blomstrand C, Eriksson PS, Nilsson M. Astrocytes and stroke: networking for survival? Neurochem Res. 2003;28:293–305. - PubMed
-
- Behrens PF, Langemann H, Strohschein R, Draeger J, Hennig J. Extracellular glutamate and other metabolites in and around RG2 rat glioma: an intracerebral microdialysis study. J Neurooncol. 2000;47:11–22. - PubMed
-
- Boido D, Farisello P, Cesca F, Ferrea E, Valtorta F, Benfenati F, Baldelli P. Cortico-hippocampal hyperexcitability in synapsin I/II/III knockout mice: age-dependency and response to the antiepileptic drug levetiracetam. Neuroscience. 2010;171:268–83. - PubMed
-
- Bordey A, Hablitz JJ, Sontheimer H. Reactive astrocytes show enhanced inwardly rectifying K+ currents in situ. Neuroreport. 2000;11:3151–3155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

