Antiangiogenic nanotherapy with lipase-labile Sn-2 fumagillin prodrug
- PMID: 22709347
- PMCID: PMC3498609
- DOI: 10.2217/nnm.12.27
Antiangiogenic nanotherapy with lipase-labile Sn-2 fumagillin prodrug
Abstract
Background: The chemical instability of antiangiogenic fumagillin, combined with its poor retention during intravascular transit, requires an innovative solution for clinical translation. We hypothesized that an Sn-2 lipase-labile fumagillin prodrug, in combination with a contact-facilitated drug delivery mechanism, could be used to address these problems.
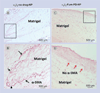
Methods: α(v)β(3)-targeted and nontargeted nanoparticles with and without fumagillin in the prodrug or native forms were evaluated in vitro and in vivo in the Matrigel™ (BD Biosciences, CA, USA) plug model of angiogenesis in mice.
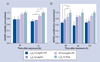
Results: In vitro experiments demonstrated that the new fumagillin prodrug decreased viability at least as efficacious as the parent compound, on an equimolar basis. In the Matrigel mouse angiogenesis model, α(v)β(3)-fumagillin prodrug decreased angiogenesis as measured by MRI (3T), while the neovasculature was unaffected with the control nanoparticles.
Conclusion: The present approach resolved the previously intractable problems of drug instability and premature release in transit to target sites.
Conflict of interest statement
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures



References
-
-
Sin N, Meng L, Wang MQ, Wen JJ, Bornmann WG, Crews CM. The anti-angiogenic agent fumagillin covalently binds and inhibits the methionine aminopeptidase, MetAP-2. Proc. Natl Acad. Sci. USA. 1997;94(12):6099–6103. ▪▪ Introduces the antiangiogenic effect of fumagillin and mechanistic insight into MetAP-2-binding.
-
-
-
Liu S, Widom J, Kemp CW, Crews CM, Clardy J. Structure of human methionine aminopeptidase-2 complexed with fumagillin. Science. 1998;282(5392):1324–1327. ▪ Structural elucidation of fumagillin complexation with human MetAP-2.
-
-
- Wang J, Lou P, Henkin J. Selective inhibition of endothelial cell proliferation by fumagillin is not due to differential expression of methionine aminopeptidases. J. Cell. Biochem. 2000;77(3):465–473. - PubMed
Patents
-
- Harris TD. M Rajopadhye: US6511648. 2003
Websites
-
- NCI. Developmental Therapeutics Program. www.dtp.nci.nih.gov.
-
- Robinson G. Histological techniques. http://mammary.nih.gov/tools/histological/histology/index.html.
Publication types
MeSH terms
Substances
Grants and funding
- NS059302/NS/NINDS NIH HHS/United States
- NS073457/NS/NINDS NIH HHS/United States
- R01 HL113392/HL/NHLBI NIH HHS/United States
- HL073646/HL/NHLBI NIH HHS/United States
- U01 NS073457/NS/NINDS NIH HHS/United States
- CA119342/CA/NCI NIH HHS/United States
- R01 HL073646/HL/NHLBI NIH HHS/United States
- HL078631/HL/NHLBI NIH HHS/United States
- R01 NS059302/NS/NINDS NIH HHS/United States
- U54 CA119342/CA/NCI NIH HHS/United States
- R01 CA154737/CA/NCI NIH HHS/United States
- N01 CO037007/AI/NIAID NIH HHS/United States
- R01 HL078631/HL/NHLBI NIH HHS/United States
- CA1547371/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
