Non-canonical signaling of the PTH receptor
- PMID: 22709554
- PMCID: PMC3428041
- DOI: 10.1016/j.tips.2012.05.004
Non-canonical signaling of the PTH receptor
Abstract
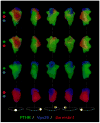
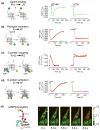
The classical model of arrestin-mediated desensitization of cell-surface G-protein-coupled receptors (GPCRs) is thought to be universal. However, this paradigm is incompatible with recent reports that the parathyroid hormone (PTH) receptor (PTHR), a crucial GPCR for bone and mineral ion metabolism, sustains G(S) activity and continues to generate cAMP for prolonged periods after ligand washout; during these periods the receptor is observed mainly in endosomes, associated with the bound ligand, G(S) and β-arrestins. In this review we discuss possible molecular mechanisms underlying sustained signaling by the PTHR, including modes of signal generation and attenuation within endosomes, as well as the biological relevance of such non-canonical signaling.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures


References
-
- Juppner H, Abou-Samra AB, Freeman M, Kong XF, Schipani E, Richards J, Kolakowski LF, Jr, Hock J, Potts JT, Jr, Kronenberg HM, et al. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science. 1991;254:1024–1026. - PubMed
-
- Schwindinger WF, Fredericks J, Watkins L, Robinson H, Bathon JM, Pines M, Suva LJ, Levine MA. Coupling of the PTH/PTHrP receptor to multiple G-proteins. Direct demonstration of receptor activation of Gs, Gq/11, and Gi(1) by [alpha-32P]GTP-gamma-azidoanilide photoaffinity labeling. Endocrine. 1998;8:201–209. - PubMed
-
- Gensure RC, Gardella TJ, Juppner H. Parathyroid hormone and parathyroid hormone-related peptide, and their receptors. Biochem Biophys Res Commun. 2005;328:666–678. - PubMed
-
- Singh AT, Gilchrist A, Voyno-Yasenetskaya T, Radeff-Huang JM, Stern PA. G alpha12/G alpha13 subunits of heterotrimeric G proteins mediate parathyroid hormone activation of phospholipase D in UMR-106 osteoblastic cells. Endocrinology. 2005;146:2171–2175. - PubMed
-
- Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, Eckhardt AE, Cowan CL, Spurney RF, Luttrell LM, Lefkowitz RJ. Distinct beta-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J Biol Chem. 2006;281:10856–10864. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

