Reducing inappropriate, anticholinergic and psychotropic drugs among older residents in assisted living facilities: study protocol for a randomized controlled trial
- PMID: 22709731
- PMCID: PMC3541247
- DOI: 10.1186/1745-6215-13-85
Reducing inappropriate, anticholinergic and psychotropic drugs among older residents in assisted living facilities: study protocol for a randomized controlled trial
Abstract
Background: Use of inappropriate drugs is common among institutionalized older people. Rigorous trials investigating the effect of the education of staff in institutionalized settings on the harm related to older people's drug treatment are still scarce. The aim of this trial is to investigate whether training professionals in assisted living facilities reduces the use of inappropriate drugs among residents and has an effect on residents' quality of life and use of health services.
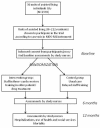
Methods and design: During years 2011 and 2012, a sample of residents in assisted living facilities in Helsinki (approximately 212) will be recruited, having offered to participate in a trial aiming to reduce their harmful drugs. Their wards will be randomized into two arms: one, those in which staff will be trained in two half-day sessions, including case studies to identify inappropriate, anticholinergic and psychotropic drugs among their residents, and two, a control group with usual care procedures and delayed training. The intervention wards will have an appointed nurse who will be responsible for taking care of the medication of the residents on her ward, and taking any problems to the consulting doctor, who will be responsible for the overall care of the patient. The trial will last for twelve months, the assessment time points will be zero, six and twelve months. The primary outcomes will be the proportion of persons using inappropriate, anticholinergic, or more than two psychotropic drugs, and the change in the mean number of inappropriate, anticholinergic and psychotropic drugs among residents. Secondary endpoints will be, for example, the change in the mean number of drugs, the proportion of residents having significant drug-drug interactions, residents' health-related quality of life (HRQOL) according to the 15D instrument, cognition according to verbal fluency and clock-drawing tests and the use and cost of health services, especially hospitalizations.
Discussion: To our knowledge, this is the first large-scale randomized trial exploring whether relatively light intervention, that is, staff training, will have an effect on reducing harmful drugs and improving QOL among institutionalized older people.
Trial registration: ACTRN12611001078943.
References
-
- Olsson J, Bergman A, Carlsten A, Oké T, Bernsten C, Schmidt IK, Fastbom J. Quality of drug prescribing in elderly people in nursing homes and special care units for dementia: a cross-sectional computerized pharmacy register analysis. Clin Drug Investig. 2010;30:289–300. doi: 10.2165/11534320-000000000-00000. - DOI - PubMed
-
- Onder G, Liperoti R, Fialova D, Topinkova E, Tosato M, Danese P, Gallo PF, Carpenter I, Finne-Soveri H, Gindin J, Bernabei R, Landi F. SHELTER project: polypharmacy in nursing home in Europe: results from the shelter study. J Gerontol A Biol Sci Med Sci. 2012;67A:698–704. doi: 10.1093/gerona/glr233. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical