Insulin-producing cells from adult human bone marrow mesenchymal stem cells control streptozotocin-induced diabetes in nude mice
- PMID: 22710060
- PMCID: PMC3893040
- DOI: 10.3727/096368912X647162
Insulin-producing cells from adult human bone marrow mesenchymal stem cells control streptozotocin-induced diabetes in nude mice
Abstract
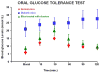
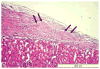
Harvesting, expansion, and directed differentiation of human bone marrow-derived mesenchymal stem cells (BM-MSCs) could provide an autologous source of surrogate β-cells that would alleviate the limitations of availability and/or allogenic rejection following pancreatic or islet transplantation. Bone marrow cells were obtained from three adult type 2 diabetic volunteers and three nondiabetic donors. After 3 days in culture, adherent MSCs were expanded for two passages. At passage 3, differentiation was carried out in a three-staged procedure. Cells were cultured in a glucose-rich medium containing several activation and growth factors. Cells were evaluated in vitro by flow cytometry, immunolabeling, RT-PCR, and human insulin and c-peptide release in responses to increasing glucose concentrations. One thousand cell clusters were inserted under the renal capsule of diabetic nude mice followed by monitoring of their diabetic status. At the end of differentiation, ∼5-10% of cells were immunofluorescent for insulin, c-peptide or glucagon; insulin, and c-peptide were coexpressed. Nanogold immunolabeling for electron microscopy demonstrated the presence of c-peptide in the rough endoplasmic reticulum. Insulin-producing cells (IPCs) expressed transcription factors and genes of pancreatic hormones similar to those expressed by pancreatic islets. There was a stepwise increase in human insulin and c-peptide release by IPCs in response to increasing glucose concentrations. Transplantation of IPCs into nude diabetic mice resulted in control of their diabetic status for 3 months. The sera of IPC-transplanted mice contained human insulin and c-peptide but negligible levels of mouse insulin. When the IPC-bearing kidneys were removed, rapid return of diabetic state was noted. BM-MSCs from diabetic and nondiabetic human subjects could be differentiated without genetic manipulation to form IPCs that, when transplanted, could maintain euglycemia in diabetic mice for 3 months. Optimization of the culture conditions are required to improve the yield of IPCs and their functional performance.
Conflict of interest statement
Conflicts of interest:
The authors disclose no Conflicts of interest
Figures








References
-
- Bilinski SM, Jaglarz MK, Dougherty MT, Kloc M. Electron microscopy, immunostaining, cytoskeleton visualization, in situ hybridization, and three-dimensional reconstruction of Xenopus occytes. Methods. 2010;51(1):11–19. - PubMed
-
- Bonner-Weir S, Deery D, Leahy JL, Weir GC. Compensatory growth of pancreatic beta-cells in adult rats after short-term glucose infusion. Diabetes. 1989;38(1):49–53. - PubMed
-
- Calne R, Ghoneim MA. Novel diabetes therapy: The seven pillars of credibility. Treatment Strategies Diabetes. Available online at: www.treatmentstrategies.co.uk./Diabetes.html.
-
- Calne RY, Gan SU, Lee KO. Stem cell and gene therapies for diabetes mellitus. Nat Rev Endocrinol. 2010;6(3):173–177. - PubMed
-
- Chatterjee AK, Sieradzki J, Schatz H. Epidermal growth factor stimulates (pro-) insulin biosynthesis and 3H-thymidine incorporation in isolated pancreatic rat islets. Horm Metab Res. 1986;18(12):873–874. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

