Osmotic stress, not aldose reductase activity, directly induces growth factors and MAPK signaling changes during sugar cataract formation
- PMID: 22710095
- PMCID: PMC3407318
- DOI: 10.1016/j.exer.2012.05.007
Osmotic stress, not aldose reductase activity, directly induces growth factors and MAPK signaling changes during sugar cataract formation
Abstract
In sugar cataract formation in rats, aldose reductase (AR) activity is not only linked to lenticular sorbitol (diabetic) or galactitol (galactosemic) formation but also to signal transduction changes, cytotoxic signals and activation of apoptosis. Using both in vitro and in vivo techniques, the interrelationship between AR activity, polyol (sorbitol and galactitol) formation, osmotic stress, growth factor induction, and cell signaling changes have been investigated. For in vitro studies, lenses from Sprague Dawley rats were cultured for up to 48 h in TC-199-bicarbonate media containing either 30 mM fructose (control), or 30 mM glucose or galactose with/without the aldose reductase inhibitors AL1576 or tolrestat, the sorbitol dehydrogenase inhibitor (SDI) CP-470,711, or 15 mM mannitol (osmotic-compensated media). For in vivo studies, lenses were obtained from streptozotocin-induced diabetic Sprague Dawley rats fed diet with/without the ARIs AL1576 or tolrestat for 10 weeks. As expected, lenses cultured in high glucose/galactose media or from untreated diabetic rats all showed a decrease in the GSH pool that was lessened by ARI treatment. Lenses either from diabetic rats or from glucose/galactose culture conditions showed increased expression of basic-FGF, TGF-β, and increased signaling through P-Akt, P-ERK1/2 and P-SAPK/JNK which were also normalized by ARIs to the expression levels observed in non-diabetic controls. Culturing rat lenses in osmotically compensated media containing 30 mM glucose or galactose did not lead to increased growth factor expression or altered signaling. These studies indicate that it is the biophysical response of the lens to osmotic stress that results in an increased intralenticular production of basic-FGF and TGF-β and the altered cytotoxic signaling that is observed during sugar cataract formation.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
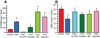

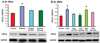
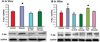

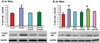
References
-
- Aberg MA, Aberg ND, Palmer TD, Alborn AM, Carlsson-Skwirut C, Bang P, Rosengren LE, Olsson T, Gage FH, Eriksson PS. IGF-I has a direct proliferative effect in adult hippocampal progenitor cells. Mol Cell Neurosci. 2003;24:23–40. - PubMed
-
- Akagi Y, Kador PF, Kinoshita JH. Immunohistochemical localization for aldose reductase in diabetic lenses. Invest Ophthalmol Vis Sci. 1987;28:163–167. - PubMed
-
- Akagi Y, Tasaka H, Terubayashi H, Kador PF, Kinoshita JH. Aldose reductase localization in rat sugar cataracts. Exerpta Medica Amsterdam. 1988
-
- Akagi Y, Terubayashi H, Millen J, Kador PF, Kinoshita JH. Aldose reductase localization in dog retinal mural cells. Curr Eye Res. 1986;5:883–886. - PubMed
-
- Akagi Y, Yajima Y, Kador PF, Kuwabara T, Kinoshita JH. Localization of aldose reductase in the human eye. Diabetes. 1984;33:562–566. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

