Calpain and MARCKS protein regulation of airway mucin secretion
- PMID: 22710197
- PMCID: PMC3486950
- DOI: 10.1016/j.pupt.2012.06.003
Calpain and MARCKS protein regulation of airway mucin secretion
Abstract
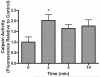
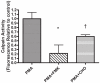
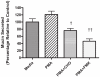
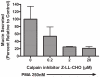
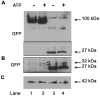
Hypersecretion of mucin plays an important role in the pathophysiology of many inflammatory airway diseases, including asthma, chronic bronchitis, and cystic fibrosis. Myristoylated alanine-rich C-kinase substrate (MARCKS) protein has been shown to play an important role in regulation of airway mucin secretion, as peptides analogous to the amino (N)-terminus of MARCKS attenuate mucin secretion by airway epithelium in vitro and in vivo. Here, we investigated a potential role for the protease Calpain, a calcium-dependent cysteine protease that can cleave MARCKS, in the MARCKS-related secretory mechanism. We theorized that Calpain might cleave MARCKS near the N-terminus, thereby attenuating the ability of MARCKS to bind to membranes and/or creating a small N-terminal peptide that could act as a competitive intracellular inhibitor to remaining endogenous full-length MARCKS molecules. Primary normal human bronchial epithelial (NHBE) cells and the virally-transformed human bronchial epithelial HBE1 cell line were exposed to phorbol-12-myristate-13-acetate (PMA) to stimulate the Protein Kinase C (PKC) pathway, leading to enhanced mucin secretion, and Calpain activity within the cells was measured with a fluorescent cleavage assay. Calpain activity was increased by PMA, and pretreatment of the cells with Calpain inhibitors reduced both Calpain activity and mucin secretion in a concentration-dependent manner. Thus, as opposed to the original hypothesis, inactivating Calpain caused a decrease rather than an increase in secretion. HBE1 cells transfected with DNA constructs encoding a MARCKS-YFP fusion protein showed cleavage at a putative site near the N-terminus in response to PMA. Cleavage of MARCKS by Calpain may have an important role in regulation of the PKC/MARCKS pathway regulating airway mucin secretion.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Protein kinase C delta regulates airway mucin secretion via phosphorylation of MARCKS protein.Am J Pathol. 2007 Dec;171(6):1822-30. doi: 10.2353/ajpath.2007.070318. Epub 2007 Nov 30. Am J Pathol. 2007. PMID: 18055557 Free PMC article.
-
MARCKS and HSP70 interactions regulate mucin secretion by human airway epithelial cells in vitro.Am J Physiol Lung Cell Mol Physiol. 2013 Apr 15;304(8):L511-8. doi: 10.1152/ajplung.00337.2012. Epub 2013 Feb 1. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23377348 Free PMC article.
-
MARCKS regulation of mucin secretion by airway epithelium in vitro: interaction with chaperones.Am J Respir Cell Mol Biol. 2008 Jul;39(1):68-76. doi: 10.1165/rcmb.2007-0139OC. Epub 2008 Feb 28. Am J Respir Cell Mol Biol. 2008. PMID: 18314541 Free PMC article.
-
Regulation of mucin secretion and inflammation in asthma: a role for MARCKS protein?Biochim Biophys Acta. 2011 Nov;1810(11):1110-3. doi: 10.1016/j.bbagen.2011.01.009. Epub 2011 Jan 31. Biochim Biophys Acta. 2011. PMID: 21281703 Free PMC article. Review.
-
Identification of myristoylated alanine-rich C kinase substrate (MARCKS) in astrocytes.Front Biosci. 2005 Jan 1;10:160-5. doi: 10.2741/1517. Print 2005 Jan 1. Front Biosci. 2005. PMID: 15574358 Review.
Cited by
-
Fibroblast Migration Is Regulated by Myristoylated Alanine-Rich C-Kinase Substrate (MARCKS) Protein.PLoS One. 2013 Jun 19;8(6):e66512. doi: 10.1371/journal.pone.0066512. Print 2013. PLoS One. 2013. PMID: 23840497 Free PMC article.
-
Targeting mucus hypersecretion: new therapeutic opportunities for COPD?Drugs. 2014 Jul;74(10):1073-89. doi: 10.1007/s40265-014-0235-3. Drugs. 2014. PMID: 24890395 Review.
-
Eosinophilic esophagitis-linked calpain 14 is an IL-13-induced protease that mediates esophageal epithelial barrier impairment.JCI Insight. 2016 Apr;1(4):e86355. doi: 10.1172/jci.insight.86355. JCI Insight. 2016. PMID: 27158675 Free PMC article.
-
Redox-Dependent Calpain Signaling in Airway and Pulmonary Vascular Remodeling in COPD.Adv Exp Med Biol. 2017;967:139-160. doi: 10.1007/978-3-319-63245-2_9. Adv Exp Med Biol. 2017. PMID: 29047085 Free PMC article.
-
Marcksb plays a key role in the secretory pathway of zebrafish Bmp2b.PLoS Genet. 2019 Sep 23;15(9):e1008306. doi: 10.1371/journal.pgen.1008306. eCollection 2019 Sep. PLoS Genet. 2019. PMID: 31545789 Free PMC article.
References
-
- Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006;86:245–78. - PubMed
-
- Li Y, Martin LD, Spizz G, Adler KB. MARCKS protein is a key molecule regulating mucin secretion by human airway epithelial cells in vitro. J Biol Chem. 2001;276:40982–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

