Diagnostic accuracy of induced sputum LAM ELISA for tuberculosis diagnosis in sputum-scarce patients
- PMID: 22710609
- PMCID: PMC5463742
- DOI: 10.5588/ijtld.11.0614
Diagnostic accuracy of induced sputum LAM ELISA for tuberculosis diagnosis in sputum-scarce patients
Abstract
Objective: To examine whether a lipoarabinomannan (LAM) enzyme-linked immunosorbent assay (ELISA) that offers diagnostic utility using urine in patients with tuberculosis (TB) and human immunodeficiency virus (HIV) co-infection can be used in induced sputum to diagnose sputum-scarce patients with suspected TB.
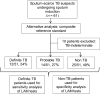
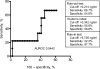
Design: LAM was measured in induced sputum samples obtained from 61 consecutively recruited sputum-scarce TB suspects in a tertiary hospital respiratory clinic in South Africa. Liquid culture positivity for Mycobacterium tuberculosis was used as the reference standard. Receiver operating characteristic analysis was used to assess alternative LAM concentration cut-offs.
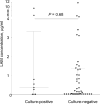
Results: A total of 87% (53/61) of study patients had a valid M. tuberculosis culture result; 49% (23/53) were HIV-infected and 17% (9/53) were culture-positive for M. tuberculosis. Induced sputum smear microscopy and LAM ELISA had an overall sensitivity of 56% (95%CI 27-81); however, the specificity of LAM ELISA was 48% (95%CI 34-62), while the positive and negative predictive values were respectively 18% (95%CI 8-36) and 84% (95%CI 65-94). An optimal rule-in cut-off selected by receiver operating characteristic (LAM concentration >5.73 ng/ml) increased test specificity to 98% and reduced sensitivity to 22%. Normalisation of the assay for sample total protein or cell count did not improve diagnostic accuracy.
Conclusions: In this proof-of-concept study, the ELISA was not clinically useful for TB diagnosis using induced sputum.
Figures



References
-
- World Health Organization. Global tuberculosis control: surveillance, planning, financing. Geneva, Switzerland: WHO; 2008. (WHO report 2008). WHO/HTM/TB/2008.393.
-
- Getahun H, Harrington M, O’Brien R, Nunn P. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet. 2007;369:2042–2049. - PubMed
-
- Tessema TA, Hamasur B, Bjun G, Svenson S, Bjorvatn B. Diagnostic evaluation of urinary lipoarabinomannan at an Ethiopian tuberculosis centre. Scand J Infect Dis. 2001;33:279–284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

